Seasons Greetings
This has been a tough year for everyone but we still need to think about those less fortunate, as usual any monies saved on cards will be donated to the MS Society. Have a safe break and let's hope for better things in 2021.

Still time to submit proposal to European Lead Factory
The closure date for the latest round of proposals for screening at the European Lead Factory is January 2021
The European Lead Factory welcomes drug targets in all therapeutic areas. Interested? Submit your screening proposal now. The next deadline to apply will be in January 2021.
Full details are on the ELF website.
I've been involved in a number of screening campaigns and I can confirm this is a high quality screening deck.
Open Source Antibiotics
Open Source Antibiotics is a consortium of researchers interested in open ways to discover and develop new, inexpensive medicines for bacterial infections.
There are already a couple of projects Mur Ligase and a series of Diarylimidazoles with unknown mechanism. Well worth a read.
All the structures of the molecules on the project are openly available in a spreadsheet https://docs.google.com/spreadsheets/d/168-a1_l51Nfbms67eG8zU8p-EhEtEO26FUzRInbu7fY/edit#gid=2078630269 feel free to have a browse.
New REVIVE Antimicrobial Encyclopaedia
Often when moving into a new therapeutic area it takes a while to pick up all the terms and acronyms, and antimicrobial research has some of it's own, not sure what a MIC is then head over to the REVIVE Antimicrobial Encyclopaedia and search.
The REVIVE Antimicrobial Encyclopaedia includes definitions of terms from the field of antimicrobials including ‘Active Pharmaceutical Ingredient’, ‘Bacterial efflux’ and ‘Minimum Inhibitory Concentration’. Each term has links for users to find more information on the subject and wherever available there are also links to REVIVE content such as webinar recordings and Antimicrobial Viewpoints on the subject. Some terms also include bespoke explanatory videos with clear diagrams featuring REVIVE experts.
REVIVE is a space for everybody with an interest in antimicrobial R&D. The Global Antibiotic Research and Development Partnership (GARDP) is a not-for-profit organization developing new treatments for drug-resistant infections that pose the greatest threat to health. We were created to ensure that everyone who needs antibiotics receives effective and affordable treatment, no matter where they live. We aim to develop five new treatments by 2025 to fight drug-resistant infections, focusing on sexually transmitted infections, sepsis in newborns and infections in hospitalized adults and children.
RSC Interest group membership
My RSC membership renewal form has just dropped though the letterbox.

It is the ideal time to think about joining one of the RSC Interest groups.
Interest groups are scientific networks run by members for their community. Each group is themed around a specific area or application of the chemical sciences. They organise an annual series of events to cater for both their members and the wider scientific community. These events vary from: multi-day conferences and workshops to training events.
If you are interested in the computational side of drug discovery why not join the Chemical Information and Computer Applications Group CICAG code 86.
Proposals for European Lead Factory
The closure date for the latest round of proposals for screening at the European Lead Factory is January 2021
The European Lead Factory welcomes drug targets in all therapeutic areas. Interested? Submit your screening proposal now. The next deadline to apply will be in January 2021.
Also note
Following the UK’s exit from the European Union on 31 January, we are pleased to confirm that the European Lead Factory still welcomes screening proposals from UK researchers.
You can read more about the screening facility here https://www.europeanleadfactory.eu/node/353.
The details of how to submit are here https://www.europeanleadfactory.eu/how-submit/drug-target-assays/how-it-works.
I've written about the ELF here.

Open Source Antibiotics
I just thought I'd share this email
Dear Friend of Open Source Antibiotics (OSA),
It's been a busy few months in OSA. Recent activity is being captured in weekly public Zoom meetings (every Friday at 2pm London at https://ucl.zoom.us/j/92800004715), and you can see the details in the recordings of those meetings (like this one) and the associated "Github Issues" (like this one).

But as part of those discussions we were wondering about the best way to update everyone quickly. While OSA uses Twitter, there is no good substitute for a short email. So this is the first, short OSA news email. Three points:
- We have guessed you're interested in receiving occasional emails about OSA. If you're not, just email us back to say you'd like to opt out. Nobody likes spam.
- Please forward this to anyone you think might be interested in antibiotics or drug discovery or open science. As an open project, everything is in the public domain, and everyone is welcome.
- To keep things short, each news email has a limit of three items. If you're interested in learning more, then each project has a wiki (current project status, like this) and the Issue Tracker (current To Do list and discussion, like this).
- We have confirmed the activity vs MRSA of the diarylimidazoles (exemplar compound OSA821 shown below), originally discovered and explored by Alvaro Lorente and Bill Zuercher at UNC Chapel Hill. A new potency screen is being performed this week at UCL by Paul Stapleton, and includes about 30 compounds that have been donated to the project via Ben Perry (DNDi). This time our potency assay will include a parallel screen of select compounds vs VRE, to see if there is activity vs other high priority Gram +ves.

- A key aim of the project is to solve the rapid clearance of the known actives. New data from Sue Charman's lab gave clues as to which compounds to investigate next, and we are finalising negotiations for some pro bono work from a UK company towards identification of possible metabolites.
- The mechanism of action of these compounds is unknown, but Lee Graves's lab at UNC are in the middle of some MIBs experiments that we hope will reveal, by the end of October, some key new insights into how the compounds work.

You can read more about the Open Source Antibiotics on GitHub https://github.com/opensourceantibiotics.
There are currently two projects MurLigase and DiarylImidazoles, everything is in the open and anyone can contribute.

Why not swing by and have a browse.
Open Targets Platform updated
Target Validation is the most critical step in the Drug Discovery process, almost everything else we can fix. Which is why the update to the Open Targets Platform is so valuable.
The latest release of the Open Targets Platform - 20.09 - is now available at https://www.targetvalidation.org/.
This release sees the addition of ClinGen to the expert curated evidence sources for rare disease genetics that includes UniProt, the Genomics England PanelApp, and Gene2Phenotype.
This latest update includes 27,610 Targets, 13,944 Diseases, 8,419,186 Evidence and 6,551,303 Associations
AI3SD Online Guest Lecture Series
Artificial Intelligence and Augmented Intelligence for Automated Investigations for Scientific Discovery (AI3SD) are running an Online Guest Lecture Series this summer. The full seminar list is here.
http://www.ai3sd.org/summer-seminar-series-2020.

If you missed a presentation or want to replay it, all the presentations are on the AI3SD YouTube channel.
RSC BMCS Hall of Fame
The RSC Biological and Medicinal Chemistry initiated a Hall of Fame a short while ago and I'd like to highlight the relevant page of the BMCS website.
The Hall of Fame is to recognise prominent chemists for outstanding, sustained, contributions to any area of interest to the BMCS, eg medicinal chemistry, agriscience, biooorganic chemistry, chemical biology. This is an Individual award to recognise prominence and significant, sustained, scientific impact in the field of medicinal chemistry, agriscience or chemical biology, including teaching excellence, outstanding contributions to the BMCS, or any combination thereof.
The first inductee to the BMCS Hall of Fame was Professor C Robin Ganellin FRS, Emeritus Professor of Medicinal Chemistry at University College London. He co-discovered histamine H2-receptors with James Black and co-invented the anti-ulcer drug cimetidine. He co-discovered butabindide, an inhibitor of the enzyme tripeptidyl peptidase II, and co-invented the histamine H3-receptor antagonist drug, pitolisant.
Cimetidine was the first histamine H2 receptor antagonist drug that inhibits stomach acid production and used in the treatment of heartburn and peptic ulcers.

Oral bioavailability is 65% and it has a half-life of 2 hours.
Butabindide is an inhibitor of the enzyme tripeptidyl peptidase II designed as an anti-obesity drug.

The histamine H3-receptor antagonist Pitolisant, is used for the treatment of excessive daytime sleepiness (EDS) in adults with narcolepsy

Pitolisant is well absorbed (90%) and has an elimination half-life of 10-12 hours.
The 2019 inductee was Sir Simon Campbell CBE FRS FMedSci who is probably best known for his work leading to Doxazosin, for high blood pressure and angina and Amlodipine – used to treat high blood pressure and prostrate enlargement.
Doxazosin is a α1-selective adrenergic blocker in the quinazoline class of compounds

Oral bioavailability is 65% and elimination half-life 22 hours, , it highly plasma protein bound (98%). Hepatic metabolism of doxazosin produces inactive O-demethylated and C-hydroxylated metabolites.
Amlodipine is a long acting calcium channel antagonist, it blocks L-type calcium channels in muscle cells and N-type calcium channels in the central nervous system.

Amlodipine is well absorbed by the oral route with a mean oral bioavailability around 60%; the half-life of amlodipine is about 30 h to 50 h, it highly plasma protein bound (97.5%). Renal elimination is the major route of excretion with about 60% of an administered dose recovered in urine, largely as inactive pyridine metabolites. Amlodipine is on the World Health Organisation's List of Essential Medicines.
Cross-referencing the Project Moonshot compounds
The project COVID moonshot is generating a significant amount of data both biochemical data distributed by PostEra and crystallographic data generated and distributed by the team at Diamond.
The COVID Moonshot is an ambitious crowdsourced initiative to accelerate the development of a COVID antiviral. We work in the open with no intellectual property constraints. This way, any scientist can view submitted drug designs and experimental data to inspire new design ideas. We use our cutting-edge machine learning tools and Folding@home's crowdsourced supercomputer to determine which drug designs to send to our partners to make and test in the lab. With each drug design tested, we get closer to our goal.
It is sometimes difficult to cross-reference compounds between multiple sources so I've downloaded the compounds with associated data calculated InChiKeys and then used the InChiKey to link compounds from different sources within Vortex. This means you have the biochemical data together with PDB code (if available) or the fragalysis code for the crystal structure. I've also annotated with identifiers from multiple databases (ChEMBL, PubChem etc.), calculated physicochemical properties (LogP/D, TPSA, HBD/A etc) and then exported in sdf format. I've also clustered the structures to aid navigation.
You can download the zipped sdf file here.
Updated. I was asked if I could provide this file in SMILES format so here it is.
I plan to try and have a look at ways to visualise the data when I can find some free time.

Precursor Chemicals list
When helping to enhance screening collections I'm sometimes asked to exclude "prohibited precursor chemicals", these are chemicals that might be used in the manufacture of illegal drugs.
The effective control of chemicals used in the illicit manufacture of narcotic drugs and psychotropic substances is an important tool in combating drug trafficking. These chemicals, known as ‘precursors’, also have legitimate commercial uses as they are legally used in a wide variety of industrial processes and consumer products, such as medicines, flavourings and fragrances.
I'm aware of this list on the UK Government website https://assets.publishing.service.gov.uk/...PRECURSORCHARTDomesticJan2014.pdf, and the listing from INCB http://www.incb.org/documents/PRECURSORS/REDLIST/2020/RedList2020E.pdf, however I'm sure it far from complete.
Does anyone know of a more complete listing? Preferably in a chemically intelligent form
The SARS-CoV-2 main protease as drug target
A very useful primer for those interested in contributing to the ongoing research efforts at COVID moonshot and Open Source COVID-19.
The SARS-CoV-2 main protease as drug target DOI
The unprecedented pandemic of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is threatening global health. The virus emerged in late 2019 and can cause a severe disease associated with significant mortality. Several vaccine development and drug discovery campaigns are underway. The SARS-CoV-2 main protease is considered a promising drug target, as it is dissimilar to human proteases. Sequence and structure of the main protease are closely related to those from other betacoronaviruses, facilitating drug discovery attempts based on previous lead compounds. Covalently binding peptidomimetics and small molecules are investigated. Various compounds show antiviral activity in infected human cells.
Remember a hit in a screen is just the very first step, there is much more to consider before it can be described as a drug candidate.
European Lead Factory Q&A
Interested in accessing a high quality high-throughput screening platform? Here is a chance to find out more about the European Lead Factory.
More details are here
The European Lead Factory (ELF) is a collaborative public-private partnership aiming to deliver novel lead molecules for drug discovery programs.
I've previously written about the ELF here.
Flatland: a nice place to be
As ever a useful analysis of the published literature on Practical Fragments, "Evaluation of 3-Dimensionality in Approved and Experimental Drug Space" DOI.
The true need for topological diversity in feedstocks and final drug molecules remains unclear given the overwhelming number of linear and planar drugs. The question remains as to whether more 3D compounds represent attractive and untapped therapeutic space, or if more linear/planar molecules are indeed the best topologies for bioactive molecules.
I came to a similar conclusion when looking at published fragment hits.
Drug Discovery Resources Website Stats
I wrote a blog entry about things that should be considered when proposing a hit identified from virtual screening as a drug candidate. Several people have suggested I create an easily identifiable web page so they can reference it. So here it is
COVID-19 and the Identification of "Drug Candidates".
I also thought I'd use the opportunity to look at the Drug Discovery Resources website stats for the first 6 months of 2020.
The Drug Discovery Resources pages are intended to act as a resource for scientists undertaking drug discovery, they were initially based on a course I give but have been expanded to give much more detail and to cover subjects not covered in the course.
The site has been viewed by almost 40,000 viewers with most people viewing a couple of pages per session. The viewers come from over 150 countries, the top countries being.
- United States (28%)
- United Kingdom (16%)
- India (9%)
- Germany (3.5%)
- China (3%)
- Canada (3%)
- Japan (3%)
The most viewed pages were
- Lipophilicity
- Calculating Physicochemical Properties
- Bioisosteres
- Distribution and Plasma Protein Binding
- Molecular Interactions
- Kinase Inhibitors
- Acid Bioisosteres
- Aromatic Bioisosteres
- Aspartic Acid Proteases
- Solvation and desolvation
- Formulation
- Fragment based screening
- Protacs
Looking at the operating systems 55% are Windows users, 20% Mac users, 12% iOS and 12% Android, Chrome dominates the browser stats (64%) with Safari second (17%) and Firefox third (7%).
Another PYMOL session file
A full-length model of glycosylated SARS-Cov-2 spike protein is recently described in literature by Casalino et. al. The PDB files for models are available at https://amarolab.ucsd.edu/covid19.php. These PDB files contain data for spike protein, glycans, lipid membrane, ions, and solvent.
Manish Sud has generated an annotated PyMol session file to view the model of spike protein present in open state conformation. The PyMOL session file is quite helpful during the reading of the article describing the work. It's a bit of elbow grease work to set up appropriate views in PyMOL and Manish has kindly shared it. It's available for download at http://www.mayachemtools.org/Download.html. I'm sure many will find it helpful.

The size of uncompressed PyMOL session file is quite large. It might take few minutes to load it into PyMOL, based on your hardware specifications.
Manish has also provided session files for SARS-CoV-2 Mpro ligands.
More COVID-19 MPro Activity Data
One of the best drug targets among coronaviruses is the main protease (Mpro), this enzyme is essential for processing the polyproteins that are translated from the viral RNA and the recognition sequence at most sites is Leu-Gln↓(Ser,Ala,Gly) and since no human enzymes have similar specificity inhibitors should be very specific. Mpro is a papain-like protease cysteine protease.
I've previously described the fragment hits from a fragment screen against crystals of the main protease (MPro) of SARS-CoV-2, the virus that causes COVID-19. Full details of the screening effort are described here https://www.diamond.ac.uk/covid-19/for-scientists/Main-protease-structure-and-XChem/Downloads.html
Additional biological results from project moonshot are now available. You can browse the data here https://postera.ai/covid/activity_data.
These results contain a significant milestone with the identification of the first sub micromolar non-covalent inhibitor.
JAG-UCB-a3ef7265-20 has been titrated twice now and has an IC50 of 0.6 uM.

This compound is a racemic mixture and the synthesis of the individual enantiomers is underway, if the activity predominantly lies with a single enantiomer we could see a further improvement in activity. The original submission was based on a pharmacophore search of Enamine based on amino-pyridine hits. I highlight this to underline the importance of simple descriptor-based searches, they are often highly competitive with sophisticated docking studies and require orders of magnitude less compute resources.
Since this research is being conducted in the public domain a number of other people have been able to contribute further ideas based on this exciting discovery.

21st RSC/SCI Cambridge MedChem Meeting
The first circular for the 21st Cambridge MedChem Meeting, 12-15th September 2021, Churchill College, Cambridge. #CamMedChem21

Full details on the website.
GARDP: Bringing new treatments for drug-resistant infections to all who need them
This webinar provided an overview and update on GARDP’s efforts to bring new antibiotic treatments for drug-resistant infections to all who need them.
The following topics were presented:
- Antibiotic resistance and the GARDP response
- Tackling the growing threat of hospital infections
- Developing new treatments for neonatal sepsis
Dexamethasone shown to reduce COVID-19 mortality
The NIHR-funded and supported study RECOVERY (Randomised Evaluation of COVid-19 thERapY) has announced that the steroid dexamethasone has been identified as the first drug to improve survival rates in certain coronavirus patients.
A total of 2104 patients were randomised to dexamethasone once per day for ten days and were compared with 4321 patients randomised to usual care alone. Among the usual care control group, 28-day mortality was highest in those on ventilators (41%), intermediate in those on oxygen only (25%), and lowest among those who were not receiving any respiratory intervention (13%).
The study, conducted at the University of Oxford and led by Professor Peter Horby and Professor Martin Landray, found that dexamethasone reduced the risk of dying by one-third in ventilated patients and by one fifth in other patients receiving oxygen only. There was no benefit among those who did not need respiratory intervention.
Dexamethasone is an inexpensive corticosteroid medication used to treat many inflammatory and autoimmune conditions, such as rheumatoid arthritis and bronchospasm. Glucocorticoids are part of the feedback mechanism in the immune system, which modulates certain aspects of immune function. They bind to the glucocorticoid receptor, and the activated complex up-regulates the expression of anti-inflammatory proteins and represses the expression of proinflammatory proteins.

Dexamethasone CHEMBL384467 has good oral bioavailability (80-90%) and a reasonable half-life (4 h), with a high volume of distribution (> 50L). It is also available as a 3.3 mg/mL solution for intravenous use. Dexamethasone is extensively metabolised to 6-hydroxydexamethasone via CYP3A4 mediated oxidation.
The oral LD50 in female mice is reported to be 6.5g/kg.
'In Silico Toxicology' Network Meeting 2020
The 'In Silico Toxicology' Network Meeting 2020 will be held on 30 September 2020, 10am-5pm (UK time).
On Zoom this year, and open to all (max 300 participants) more details and registration here http://www.drugdiscovery.net/tox2020/.
An event (with free registration) to foster the In silico Toxicology Community in the UK and beyond. Scientific contributions are welcome as are those on ongoing work, regulatory aspects, industry perspectives, databases, relevant software, etc. in the field. This event is meant to stimulate interactions and discussions, hence speakers are asked to present both about successes and applications that work, as well as areas where still further work is needed, in order to truly develop the field in the future.
Emerging Technologies Competition
The Emerging Technologies Competition is the Royal Society of Chemistry’s annual initiative for early stage companies and academic entrepreneurs who want to commercialise their technologies to make a global impact.
They are seeking applications from entrepreneurs who are developing technologies that have a strong chemistry component and fall within one of the following categories:
- Health
- Food & Drink
- Energy & Environment
- Enabling Technologies
From the application round, 24 finalists are selected to present their technologies to a panel of judges. These judges then choose 4 winners (1 per category). Winners receive £20,000 and mentorship. Applications close 12 July 2020.
There are more details here https://www.rsc.org/competitions/emerging-technologies/
Fragments and novelty
I've spoken to a couple of people recently who are very focused on identifying novel fragments for their fragment screening collection. I have to say I'm not convinced about the benefit of populating your fragment collection with novel fragments. One of the really attractive features of fragment-based screening is the ability to follow up and verify the initial round of fragment hits by testing commercially available analogues, or isomers.
You can read more here Fragments and novelty.
Crystallographic and electrophilic fragment screening of the SARS-CoV-2 main protease
Full details of the Crystallographic and electrophilic fragment screening of the SARS-CoV-2 main protease are now published. https://www.biorxiv.org/content/10.1101/2020.05.27.118117v1.full.pdf
An extraordinary effort highlighted by the timeline shown below.

The results of the first round of biological results from project moonshot are in. You can browse the data here https://postera.ai/covid/activity_data.
A listing of my posts on COVID-19 are here.
First round of MPro bioactivity results
One of the best drug targets among coronaviruses is the main protease (Mpro), this enzyme is essential for processing the polyproteins that are translated from the viral RNA and the recognition sequence at most sites is Leu-Gln↓(Ser,Ala,Gly) and since no human enzymes have similar specificity inhibitors should be very specific. Mpro is a papain-like protease cysteine protease
I've previously described the fragment hits from a fragment screen against crystals of the main protease (MPro) of SARS-CoV-2, the virus that causes COVID-19. Full details of the screening effort are described here https://www.diamond.ac.uk/covid-19/for-scientists/Main-protease-structure-and-XChem/Downloads.html
The results of the first round of biological results from project moonshot are in. You can browse the data here https://postera.ai/covid/activity_data.

Most of the most active compounds are chloroketones or acrylamides, presumably covalent inhibitors, and they all show selectivity over Trypsin (IC50 >99 uM).
There are a few structures that look more like competitive inhibitors shown below

A number of these structures now have crystal structures available.
A sdf file containing these non-covalent structures is here
Fantastic work by all involved.
COVID-19 and the Identification of "Drug Candidates"
One of the really heartening things to come out of the current pandemic is the willingness of many scientists to put aside their own research and throw themselves into the efforts to find a treatment. However, lack of domain expertise is always a problem when scientists enter a new field, so I thought I'd put together a few things to consider.
In silico screening, for docking experiments you need to put considerable effort into ensuring the protein structure used is appropriate, you can't simply download a PDB file from the Protein Data Bank and use it. It will undoubtedly contain errors, you will need check protonation, hydrogen bonds etc. Then there is the issue of deciding which solvent molecules are important. Binding energies, docking scores are not as accurate as many seem to assume and no substitute for an experienced medicinal chemists looking at the bound poses, I've tried to summarise the types of molecular interactions here. Remember to also think about the impact of solvation. For other virtual screening approaches you need to be very careful about the quality of the input data. In many cases it will be heavily biased towards actives.
In silico predictions are no substitute for biological data, if you are using repurposed drugs or available chemicals there is really no excuse for not generating the appropriate in vitro biological data, there are many labs who would be happy to collaborate. If the molecules are novel many custom synthesis companies have offered to help. Remember that the IC50 is probably not that useful, it is likely that you will want to block the target 100% so you need to be above the IC95. In vitro biochemical assays using isolated enzymes will often give a false sense of potency, you should also determine activity in a cell-based assay in the presence of plasma.
If you are proposing a repurposed drug there will be a lot of information about the drug in the public domain, you may well need to search for compound codes, and various drug name synonyms. UniChem is a very useful web service for cross-referencing between chemical structure identifiers.
There are now many free, web-accessible databases some useful starting points are shown in the table below.
| Name | Link | Description |
|---|---|---|
| ChEMBL | https://www.ebi.ac.uk/chembl/ | A database of bioactive drug-like small molecules, it contains 2-D structures, calculated properties (e.g. logP, Molecular Weight, Lipinski Parameters, etc.) and abstracted bioactivities (e.g. binding constants, pharmacology and ADMET data). |
| PubChem | https://pubchem.ncbi.nlm.nih.gov | Three linked databases within the NCBI's Entrez information retrieval system. These are PubChem Substance, PubChem Compound, and PubChem BioAssay. Many compounds have links to primary literature and patents |
| Guide to Pharmacology | https://www.guidetopharmacology.org/GRAC/searchPage.jsp | An expert-driven guide to pharmacological targets and the substances that act on them. |
| DrugBank | https://www.drugbank.ca | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug data with comprehensive drug target information |
| NCI Thesaurus | https://ncithesaurus.nci.nih.gov/ncitbrowser/ | NCI Thesaurus (NCIt) provides reference terminology for many NCI and other systems. It covers vocabulary for clinical care, translational and basic research, and public information and administrative activities |
| Clinical Trials | https://clinicaltrials.gov | A database of privately and publicly funded clinical studies conducted around the world |
| FDA | https://www.fda.gov | Food and Drug Administration responsible for safety and efficacy of drugs |
| WIPO | https://www.wipo.int/portal/en/index.html | World IP services |
Find out the original target and mode of action. I've seen a couple of proposed compounds that are known prodrugs, the parent compound is designed to either breakdown or be modified in vivo to yield the active compound. The prodrug may have negligible systemic exposure. Covalent modifiers may look attractive but selectivity is always a concern and they may have narrow therapeutic windows.
Look at the original indication, many anticancer drugs are extremely toxic and could not be given other patients. Similarly, drugs that reduce blood pressure or other physiological changes may be problematic. You may well be able to find counter-screening data, this could highlight problematic off-target activities.
Look at the approved dosing regime, if a drug is only approved for doses of 2 ug/kg there might well be good reasons, and if your proposed drug only has uM activity in the in vitro assays you won't be able to generate sufficient plasma concentrations. Check what safety studies have been undertaken, are they sufficient to support multi-day dosing?
Look at the pharmacokinetics, you should be able to model the dosing regime needed to maintain plasma concentrations above IC95, this will may need to be maintained 24 hours a day. Check protein binding and distribution and use in the predictive modelling.

Look for the routes of administration, for in intensive care I suspect many will need the drug to be administered i.v. if there is no intravenous formulation is the drug soluble enough for one to be developed, ber in mind the limitations of intravenous formulations
Many of the patients will be on multiple drugs, both to treat the viral infection but also adventitious bacterial infections and since many are elderly and have pre-existing medical conditions they may have a cocktail of drugs prescribed. Drug-Drug interactions thus become a major concern, any proposed drug to treat the virus that has major interactions with CYP450 enzymes (induction, inhibition or metabolism) is likely to hugely complicate the overall dosing regime.
Check for any toxicity information, particularly black box warnings. HERG inhibition and QT prolongation is an issue that most drug discovery projects have to address at some point. This is particularly worrying if coupled with potential drug-drug interaction described above. You should also be able to find the data from safety studies, these may describe the dose limiting toxicities.
All of this information should be in the public domain, and if you are proposing a compound as a "Drug Candidate" you should not be expecting someone else to pull it all together to decide whether it is worth pursuing clinically.
Updated 26 April 2020
This Week in Virology
An interesting weekly podcast that is currently topical.
This week Doris Cully joins TWiV to discuss inhibition of SARS-CoV-2 in cell culture by ivermectin.
COVID-19 Registered Trials
There are now a number of clinical trials underway and this review by The Centre for Evidence-Based Medicine provides an excellent summary of the trials that are taking place. They describe proposed pharmacological interventions and their mechanisms, when known, but unfortunately don't give the chemical structures.
Updated
I've also now included a few other structures that people have sent to me.
Here is the workflow I use to get the structures and access more information about the compounds.
Create a text file with all the structures mentioned
ASC09
Azvudine
Azithromycin
Baloxavir
Carriomycin
Chloroquine
cobicistat
Danoprevir
Darunavir
Dihydroartemisinin
Favipiravir
Fingolimod
hydroxychloroquine
Jakotinib
Leflunomide
Lopinavir
marboxil
Methylprednisolone
oseltamivir
piperaquine
Remdesivir
ribavirin
ritonavir
Ruxolitinib
Suramin
Thalidomide
thymosin
Triazavirin
Umifenovir
Now read the text file into Vortex

The use a Name to Structure script to use a web service to get the structures, in this case I used ChemSpider. Now generate the InChiKey from the structures.

We can now use the InChiKey to search UniChem using another Vortex script to get identifiers for the molecule from various databases.
UniChem efficiently produces cross-references between chemical structure identifiers from different databases

We can then use the identifiers to search the various databases for more information
I've been asked if I could provide the structures for download
Here it is in SDF file format http://cambridgemedchemconsulting.com/news/files/COVID19/coviddata.sdf.zip
And in SMILES format http://cambridgemedchemconsulting.com/news/files/COVID19/forpost.smi.zip.
The quality of the crystal structure is critical
Crystal structures are not perfect, and it is important to understand the limitations and not assume as Derek Lowe once put it, they are a "message from God". It might be worth reading the section on structure-based design.
With this in mind I thought I'd flag this message from Bobby Glen (Cambridge) here.
Hi, we’re still (Jason at CCDC) porting GOLD to our HPC system so we can basically parallel dock. We should be able to dock and score early next week I hope, There are a few issues we also are addressing wrt the crystal structures, Gerard Bricogne at Global Phasing is kindly re-refining the published structure from the ED, this hopefully will inform us of for making some changes to the orientation/pKa and tautomers of the histidines and some of the other AAs. It’s very difficult to ‘see’ hydrogen in x-ray and these are inferred from the structure. We need to be sure we have a decent model of this (at physiological pH) before doing all the calculations. An example is H163, which is in the binding site, and is critical to a few of the interactions seen in ligands for this class of proteases. Automated hydrogen addition can be problematic.
News categories Updated
The News Categories provides a means to rapidly find new articles and update information on this site.
There has been a significant increase in several categories so I've updated the page.
For example:
Help design inhibitors of the SARS-CoV-2 main protease
Are you a medicinal chemist currently locked out of your lab?
Why not take a break from writing papers/reports and lend your expertise to this effort, https://covid.postera.ai/covid. They have identified 60 fragment hits and are asking for insight in what should be made next.
We are now asking for your help in designing new inhibitors based on these initial fragment hits: the exceptionally dense readout suggests countless opportunities for growing and merging, and we need many sharp brains to sift through them; it is also what makes us believe that potency can be directly achieved.
The first round of submissions will be reviewed tonight and the selected molecules will be made by Enamine.
Structures of SARS-CoV-2 ligands PYMOL session files
One of the best drug targets among coronaviruses is the main protease (Mpro), this enzyme is essential for processing the polyproteins that are translated from the viral RNA and the recognition sequence at most sites is Leu-Gln↓(Ser,Ala,Gly) and since no human enzymes have similar specificity inhibitors should be very specific. Mpro is a papain-like protease cysteine protease
I've previously described the fragment hits from a fragment screen against crystals of the main protease (MPro) of SARS-CoV-2, the virus that causes COVID-19. Full details of the screening effort are described here https://www.diamond.ac.uk/covid-19/for-scientists/Main-protease-structure-and-XChem/Downloads.html
I've downloaded all the structures that were screened, both those that bind and those where no binding was observed and put them into a single file, also added inChiKey, SMILES, PubChem ID, PDB ID of ligand if known and a range of other identifiers from different databases, the file is available here http://cambridgemedchemconsulting.com/news/files/Archive.zip.
Whilst that is probably sufficient for those looking at cheminformatics driven approaches to designing new molecules anyone wanting to undertake structure based design would need to download all the structures and then overlay them to visualise on their desktop. Fortunately Manish Sud of MayaChemTools has done the hard work and generated a series of PYMOL session files that allow you explore the enzyme crystal structure and the screening data interactively.
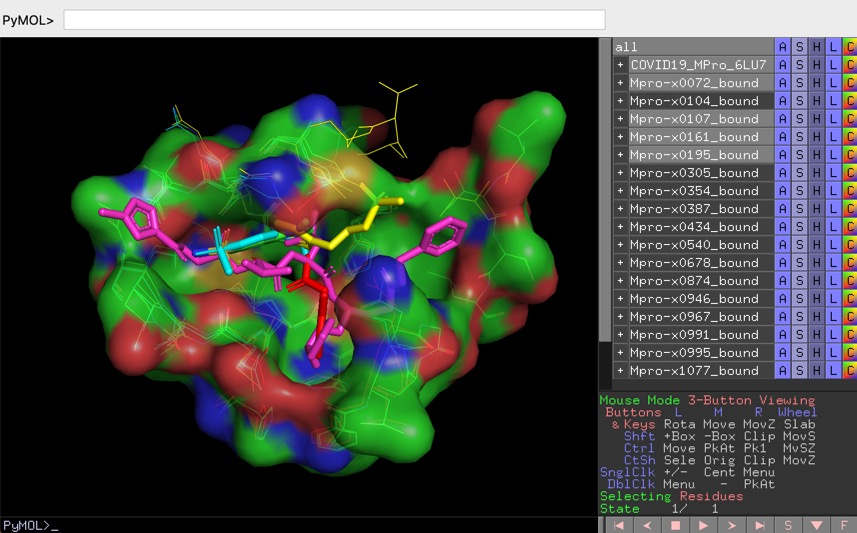
PYMOL is an open source molecular visualisation application, you can download it here https://pymol.org/2/ or install using conda
conda install -c schrodinger pymol
If you have not used it before there is a tutorial here
The PyMOL session files are setup to facilitate the analysis of protein ligand interactions in the binding pocket, to view the files select "Open" from the file menu bar, some of the larger files make take a little while to load.
X-ray crystal structures and electron densities
COVID-19 main protease with unliganded active site (2019-nCoV, coronavirus disease 2019, SARS-CoV-2) 6Y84 and the crystal structure of COVID-19 main protease in complex with an inhibitor N3 6LU7.
The PYMOL session files (zipped) can be downloaded here
http://cambridgemedchemconsulting.com/news/files/COVID19/COVID19-MPro-6LU7-6Y84.pse.zip
Structures for non-covalent ligands
The structures of the non-covalent ligands are here.
If you are not familiar with fragment-based screening there is an introduction here including some examples of fragment growing.
It is likely that fragments will only have very modest affinity and that to completely suppress the enzyme it will require very high affinity ligands with good pharmacokinetics to achieve 100% occupancy for 24 hours per day. For this reason molecules that irreversibly bind to the enzyme might be an attractive alternative option.
Structures for covalent ligands are here
The session file containing the covalent ligands are here
This is a large file so Manish has divided it.
Whilst much of drug discovery deals with non-covalent, reversible interactions with the target protein there are also a class of therapeutic agents that bind covalently to the target protein, these are described on this page. To mitigate the risk of off-target toxicity you will need to maximise the selectivity for the target enzyme. Glutathione conjugation can be used as a surrogate for off-target reactivity.
Getting designs made
Once you have designed a novel ligand have a look at Design a Compound, We Will Make It
Designs will be prioritized by factors, such as ease of synthesis, and toxicity modeling, then synthesized by Enamine and tested by groups around the world. PostEra will be running machine learning algorithms in the background to triage suggestions and generate synthesis plans to enable a rapid turnaround. You will be informed of the progress of the molecules through the main stages (validation, synthesis and testing).
COVID-19 Open Research Dataset Challenge (CORD-19)
There are a number of COVID-19 Kaggle challenges open at the moment, https://www.kaggle.com/datasets?search=COVID.
One of the more recent is:-
COVID-19 Open Research Dataset Challenge (CORD-19)
There is a large body of research and literature continuously evolving around COVID-19. Help the research community and global organizations better digest this to answer key questions."
In response to the COVID-19 pandemic, the White House and a coalition of leading research groups have prepared the COVID-19 Open Research Dataset (CORD-19). CORD-19 is a resource of over 29,000 scholarly articles, including over 13,000 with full text, about COVID-19, SARS-CoV-2, and related coronaviruses. This freely available dataset is provided to the global research community to apply recent advances in natural language processing and other AI techniques to generate new insights in support of the ongoing fight against this infectious disease. There is a growing urgency for these approaches because of the rapid acceleration in new coronavirus literature, making it difficult for the medical research community to keep up.
You can read more about it here
favipiravir shows promise in treatment of COVID-19
Favipiravir, also known as T-705, Avigan, or favilavir is a drug designed to treat RNA viral infections DOI and DOI. It is phosphoribosylated by cellular enzymes to its active form, favipiravir-ribofuranosyl-5'-triphosphate (RTP) and inhibits the RNA-dependent RNA polymerase.

Favipiravir has recently been reported to be effective in the treatment of coronavirus patients Link. It appears to be effective in patients showing mild to moderate symptoms, but not effective in patients showing more severe symptoms.
A search of UniChem using the InChiKey gives details of all identifiers and links to clinical studies.
A number of clinical trials have been completed or are ongoing on ClinicalTrials.gov and can be found here.
Whilst it appears to be safe and well tolerated, and it has been approved for flu it has not yet been approved for COVID-19,
Fragment hits for SARS-CoV-2
A group of researchers including Dave Stuart, Martin Walsh, and Frank von Delft (Diamond Light Source) has performed a fragment screen against crystals of the main protease (MPro) of SARS-CoV-2, the virus that causes COVID-19. Even before fully analyzing all of the data they have released interim results https://www.diamond.ac.uk/covid-19/for-scientists/Main-protease-structure-and-XChem.html.
The hits can be viewed on fraglaysis here.
I've downloaded all the structures that were screened, both those that bind and those where no binding was observed and put them into a single file, also added inChiKey, SMILES, PubChem ID, PDB ID of ligand if known and a range of other identifiers from different databases, the file is available here http://cambridgemedchemconsulting.com/news/files/Archive.zip

ChEMBL 26 Released
The latest release of the essential molecule bioactivity dataset has just been announced.
ChEMBL 26 contains
- 2,425,876 compound records
- 1,950,765 compounds (of which 1,940,733 have mol files)
- 15,996,368 activities
- 1,221,311 assays
- 13,377 targets
- 76,076 documents
A couple of notes
We are now using RDKit for almost all of our compound-related processing. For the first time in ChEMBL26, this will include compound standardization, salt-stripping, generation of canonical smiles, structural alerts, image depiction, substructure searches and similarity searches (via FPSim2: https://github.com/chembl/FPSim2). Therefore, all molecules have been reprocessed and you may notice some differences in molfiles, smiles and structure search results compared with previous releases. The ChEMBL structure curation pipeline has been released as an open source package: https://github.com/chembl/ChEMBLStructure_Pipeline, and incorporated into our Beaker web services (see below). More information can be found here: http://chembl.blogspot.com/2020/02/chembl-compound-curation-pipeline.html.
We are also now using ChemAxon tools to calculate most acidic and basic pKa, logP and logD (pH 7.4) predictions, rather than ACDLabs software. These properties have therefore been recalculated and renamed in the database.
The release notes contain more details and the database can be downloaded from the ChEMBL FTP site.
Discovery of novel antibiotic Halicin using deep learning
A recent paper has caught a lot of attention recently "A Deep Learning Approach to Antibiotic Discovery" DOI from Regina Barzilay's group at MIT. They used a deep neural network model to predict growth inhibition of Escherichia coli using a collection of 2,335 molecules, the molecules were described using Morgan fingerprints, computed using RDKit, for each molecule using a radius of 2 and 2048-bit fingerprint vectors. Using this methodology they identified the known c-Jun N-terminal kinase inhibitor SU3327 which they renamed Halicin. A quick search using MolSeeker allowed identification of the structure and inChiKey.

A search of UniChem using the InChikey NQQBNZBOOHHVQP-UHFFFAOYSA-N identified a number of other identifiers in different databases.

Including a link to the ChEMBL entry CHEMBL510038 giving the biological data 0.7 nM Inhibition of c-Jun N-terminal kinase by time-resolved FRET assay, and links to the original 2009 publication DOI describing the c-JNK SAR. The compound has a rat half-life of 0.45 h. There is another publication that might be of interest describing "Discovery of 2-(5-nitrothiazol-2-ylthio)benzo[d]thiazoles as novel c-Jun N-terminal kinase inhibitors" DOI.
Certainly an interesting approach, I suspect the nitrothiazole functionality would set off a few structural alerts but there are certainly of plenty of similar compounds commercially available that would allow exploration of the SAR without too much investment in resources.
All code and data is available on GitHub and there is also a website where you can test your own molecules http://chemprop.csail.mit.edu.

Potential 2019-nCoV 3C-like Protease Inhibitors
Chris Southan recently flagged a number of publications describing possible treatments for 2019-nCoV using repurposed existing drugs "Therapeutic options for the 2019 novel coronavirus (2019-nCoV)" DOI. In addition a recent preprint "Potential 2019-nCoV 3C-like Protease Inhibitors Designed Using Generative Deep Learning Approaches" DOI highlighted the design of potential protease inhibitors. The authors provide the structures of the molecules in the supplementary informations.
I downloaded the sdf file and used a Jupyter notebook to calculate a range of physicochemical properties, the results are shown in the plot below.

As often seen with protease inhibitors, the molecular weight is rather high with the majority of compounds having Mol Weight >500. The calculated LogP is mainly in the range 2 to 5, however because 40% of the molecules are predicted to be basic the LogD is mainly in the range 0-4. This combination of high molecular weight and rather high LogP is likely to compromise the developability score (for more details on develop ability score read "20 years Rule of Five" report here.

The molecules are also predicted to have a rather high number of hydrogen bond donors and acceptors, this contributes to high polar surface area (TPSA). In general TPSA >120 are often associated with poor oral bioavailability, however it should be noted that the TPSA was not calculated on a 3D structure and it is possible that intramolecular hydrogen bonds may reduce the actual TPSA, also described in the 20 years Rule of Five report.
Scanning through the molecules I noticed a number of functional groups that might be a concern (e.g. Micheal Acceptors), I ran a couple of Vortex scripts that flag potential problematic groups based on SMARTS taken from the following publications
http://pubs.acs.org/doi/abs/10.1021/jm901137j
http://pubs.acs.org/doi/abs/10.1021/jm5019093
https://doi.org/10.1177/1087057116639992
https://dx.doi.org/10.1177%2F1087057113516861
I also ran a couple scripts that flag potential liver toxicity or HERG liabilities. These flags should not be used to exclude molecules but should be used to flag molecules for checking experimentally. The script identifies the functional group that has been flagged as a liver toxicity liability, and identifies the most similar molecule in ChEMBL that has HERG activity. The results are shown in the image below.

I also added InChiKeys for better cross referencing.
I've exported all results as an sdf file which can be found here http://cambridgemedchemconsulting.com/news/files/INSCoV_2020sdfv1addedflags.sdf.zip.
SCI-RSC Workshop on Computational Tools for Drug Discovery 2020
The SCI Fine Chemicals Group and RSC Chemical Information and Computer Applications Group are organising a second Workshop on Computational Tools for Drug Discovery. The meeting format will be the same as the very successful meeting run in Birmingham in 2019.
The 2020 workshop will be held on 19 May 2020 at Riverside West, Whitehall Road, Leeds , West Yorkshire, LS1 4AW.

The 2020 workshop will be held on 19 May 2020 at Riverside West, Whitehall Road, Leeds , West Yorkshire, LS1 4AW,
The Workshop Providers and Facilitators are
- Al Dossetter, MedChemica
- Greg Landrum, KNIME
- Gunther Stahl, OpenEye
- Ilenia Giangreco, CCDC
- Matt Segall, Optibrium
- Stuart Firth-Clark, Cresset
Attendees will be able to choose from 4 of 6 sessions.
To select which workshops you would like to attend for each session, please complete the survey on the website. Please note that spaces are allocated on a first-come, first-served basis.
More details of the workshops and registration details are on the website shown below
https://www.soci.org/events/scirsc-workshop-on-computational-tools-for-drug-discovery.
Who’s sharing their clinical trial results?
An interesting recent publication "Compliance with legal requirement to report clinical trial results on ClinicalTrials.gov: a cohort study" DOI has highlighted the failure of many institutions to report the results of clinical trials within 1 year of completion as required by law.
4209 trials were due to report results; 1722 (40·9%; 95% CI 39·4–42·2) did so within the 1-year deadline. 2686 (63·8%; 62·4–65·3) trials had results submitted at any time. Compliance has not improved since July, 2018.
Thus nearly 60% of trials are not reported within the deadline, they also looked at the relative compliance of the different sectors
Industry sponsors and large (experienced) sponsors were most likely to report trial data, whereas universities were the least likely. The sponsor with the lowest compliance was the US government.
To aid monitoring they have produced FDAAA trials Tracker which allows anyone to check compliance.

31st symposium on Medicinal Chemistry in Eastern England
The Symposium on Medicinal Chemistry in Eastern England, known colloquially as the "Hatfield MedChem" meeting, is a highly successful, long-standing, one-day meeting which runs annually. The scientific program comprises of presentations showcasing medicinal chemistry case studies from tools to candidates, across a range of modalities, therapeutic areas and target classes, as well as covering more general topics from the forefront of drug discovery of relevance to medicinal chemists. The meeting aims to be informal and interactive and the event will offer excellent scientific and networking opportunities for all those working in medicinal chemistry and drug discovery.
It will take place on Thursday 30th April 2020 at The Fielder Centre, Hatfield, Hertfordshire, UK
Registration is now open.
Full details of the scientific programme and registration details are on the website https://www.maggichurchouseevents.co.uk/bmcs/hatfield_symposium-31.htm
Always a very popular meeting so registration early is recommended.
Twitter hashtag #HatfieldMedChem20
Phenotypic Screening now offered by the European Lead Factory
The European Lead Factory has announced that it can now offer two types of phenotypic screening:
- A high-throughput, but “lower content” phenotypic approach that is suited to screening ELF’s entire compound collection, and
- A more complex “high content” screening approach using microscopy or flow cytometry to probe phenotype on a smaller subset of the compound collection
While low content assays can be live measurements or have fixed end points and involve well-averaged readouts, high content assays can be much more complex, based on live or fixed cells, multiple cell types and usually have more than one parameter as a readout. The complexity of the latter workflow makes it better suited to being performed on a smaller representative subset of the large collection.
Phenotypic screening historically has been the basis for the discovery of many drugs. Compounds are screened in cellular or animal disease models to identify compounds that cause a desirable change in phenotype. Only after the compounds have been discovered are efforts made to determine the biological targets of the compounds - a process known as target deconvolution.
Proposals for phenotypic screening approaches follow the normal review and selection process. A dedicated application form is available here.
The submission deadline for the next review and selection round is February 7, 2020.
EFMC Prize for a Young Medicinal Chemist in Industry/Academia
I just thought I'd highlight this award.
The EFMC created the “EFMC Prize for a Young Medicinal Chemist in Industry/Academia” as we felt it was important to acknowledge and recognise outstanding young medicinal chemists (≤ 12 years after PhD) working in European industry and academia. The 2020 Prizes will be given at the XXVI EFMC "International Symposium on Medicinal Chemistry" (EFMC-ISMC 2020) to be held in Basel, Switzerland on September 6-10, 2020. Both prizes consist of a diploma, an invitation to give an oral communication at the EFMC-ISMC, and a cash prize of € 1,000.
To find out more on the regulations and the application procedure visit the EFMC website: https://www.efmc.info/prizes, closing date Jan 31 2020.
Drugs approved by EMA in 2019
I recently posted details of the small molecule drugs approved by the FDA in 2019. This generated considerable interest and I thought it might worthwhile doing a similar thing for the drug approvals in Europe. However this turns out to be less straight-forward, medicines can be authorised in several European countries simultaneously by using one of three procedures: the 'centralised procedure', the 'mutual-recognition procedure' or the 'decentralised procedure'. Medicines can also be authorised in a single Member State by using the national authorisation procedure of that country. The European Medicines Agency is responsible for the centralised procedure so I downloaded just the drugs approved via this mechanism.
Of the 61 approvals in 2019, 45 were small molecule drugs and 16 were biologics. The structures of the small molecules are shown below

Looking at the calculated physiochemical properties of the small molecules one thing is quite interesting, around 50% are predicted to be ionised at physiological pH.
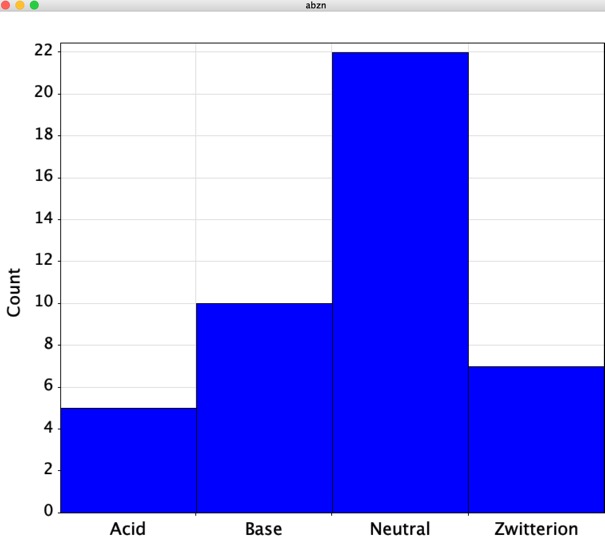
As shown in the plot below (Blue = small molecules, Green = Biologics) the largest group of drugs were Antineoplastic agents, the next largest groups being anti-virals and immunosuppressants.

Biosimilars
Four "biosimilars" were also approved. Kromeya and idacio both of which contain adalimumab (Humira) a TNF-alpha inhibitor as the active ingredient. Adalimumab was the first fully human monoclonal antibody approved by the FDA in 2002.
Grasustek contains the active substance pegfilgrastim (Neulasta) a PEGylated form of the recombinant human granulocyte colony-stimulating factor (GCSF) analog filgrastim. Zirabev contains the active substance bevacizumab (Avastin) that blocks angiogenesis by inhibiting vascular endothelial growth factor A (VEGF-A).
These four drugs join a number of other biosimilars approved in Europe, with the UK in particular keen to move to biosimilars. Biosimilars are expected to save the EU up to $44 billion in health care costs by 2020 LINK.
Most important, the EU is realizing the benefits of biosimilars without sacrificing safety or quality. Of the biosimilars approved since 2006, none have been withdrawn or suspended for safety or efficacy reasons. Further, regulators have not identified any differences in the nature, severity or frequency of adverse effects between biosimilars and biologics.
Fragment based screening pages updated
I spent some time over the Christmas break updating the Drug Discovery Resources pages on Fragment-Based screening, adding new vendors and updating the physicochemical profiles. I've also added some discussion on the elaboration/optimisation of fragments.
The pages are
Fragment-Based Screening
Building a Fragment Collection
Available Fragment Collections
Profiles of Fragment Collections
Fragment-Based Screening Published Hits
The published fragments contains details of fragments that have been reported as hits in the literature, this database now has over 1500 entries culled from over 310 publications directed at nearly 220 different molecular targets using 26 different detection technologies.

It could be argued that published fragment hits perhaps gives us an insight into the best fragments to include in library design.
