Small molecules approved by FDA in 2019
Drug approvals from the FDA in 2019 a total of 48 with the "small" molecules shown below.

Calculated physicochemical properties for the individual components (I guess some are not so small).

Seasons Greetings
Have a great time and a successful New Year, it's a beautiful world let's make sure it is still there for future generations.

As usual any monies saved on cards will be donated to the MS Society
LogP/D page updated
The page on LogP and LogD is one of the most frequently read and it has been updated to include recent publications.
Lipophilicity is possibly the most important physicochemical property of a potential drug, it plays a role in solubility, absorption, membrane penetration, plasma protein binding, distribution, CNS penetration and partitioning into other tissues or organs such as the liver and has an impact on the routes of clearance. It is important in ligand recognition, not only to the target protein but also CYP450 interactions, HERG binding, and PXR mediated enzyme induction.
The contributions of various functional groups to LogD has been explored "LogD contributions of substituents commonly used in medicinal chemistry" DOI, this study used matched molecular pairs analysis of experimental LogD values from several thousand compounds collected using the shake-flask method at pH = 7.4. They reported the average deltaLogD difference for particular molecular pairs. I've compared these experimental results with calculated LogD.

ADME pages updated
I've spent a while updating the ADME section of the Drug Discovery Resources. I particular I've added a little on the Developability score DOI that identifies four distinct cLog P/molecular weight regions that define optimal and sub-optimal chemical space. I've also added a couple of useful references.
In addition, I've expanded the Absorption and Bioavailability page to include more on bioavailability with links to physicochemical properties. The Distribution and Plasma Protein Binding section has a couple of extra examples demonstrating the impact plasma protein binding has on other pharmacokinetic properties. I've added a few details of in vitro assays to the Transporters page, and expanded the in silico brain penetration models section.
The section on Aldehyde oxidase has been greatly expanded and now includes a section on prediction and mitigation, and added useful references.

BioBlocks
I've added a new entry on to the available fragments page, BioBlocks is a newcomer to the field of fragment collections. Whilst many collections are culled from available compound collections using calculated property filters (eg Rule of 3), BioBlocks have designed novel fragments and as such there is negligible overlap with other collections. One concern with bespoke fragments is that it is often a challenge to find related analogues for followup. The BioBlocks Comprehensive Fragment Library (CFL) is a subset generated from a >1 million member synthesizable virtual library, so follow up compounds can be generated using proven in house chemistry.

Practical Fragments Poll
The latest fragment-finding methods poll has been published on Practical Fragments.
The results underline the increase in the use of fragment based screening across the industry with 85% of the respondents now reporting that they actively use fragment screening. The technologies used to detect binding have also diversified with X-ray, NMR and SPR dominating. This mirrors my findings from published fragment hits. The choice of detection technology may be due to the additional structural information that X-Ray and NMR can offer.

I was delighted to see this comment,
For the first time we asked about use of literature to identify fragments, and nearly a third of respondents said they incorporate previously published fragments into their work. As the amount of publicly available information continues to increase it will be interesting to see whether this number grows.
I'll be updating the published fragment hits at the end of the year.
OpenTargets updated
Just had an email about the latest Open Targets Platform release - 19.11.
In this release there is data on
- 27,069 targets
- 13,579 diseases
- 8.91 million pieces of evidence
- 6.33 million associations between targets and diseases
20 Years of Rule of 5 Meeting Report
It has been over twenty years since Lipinski published his work determining the properties of drug molecules associated with good solubility and permeability. Since then, there have been a number of additions and expansions to these “rules”. There has also been keen interest in the application of these guidelines in the drug discovery process and how these apply to new emerging chemical structures such as macrocycles. This symposium brought together researchers from a number of different areas of drug discovery and provided a historical overview of the use of Lipinski’s rules, as well as looking to the future and how we use these rules in the changing drug compound landscape.

The 20 Years of the Rule of Five Meeting brought together researchers from a number of different areas of drug discovery and provided both a historical overview of the use of Lipinski’s rules, as well as looking to the future and how these rules might evolve in the changing drug compound landscape. The meeting had a capacity attendance of over 100, with Sygnature kindly providing the venue. The audience was a nice mix of industry “veterans”, students and those new to the industry. The meeting format was a morning session giving a historical viewpoint followed by a panel discussion, and the afternoon was dedicated by a more forward looking session again followed by a panel discussion.
The full report is here in PDF format Full Report, many thanks to the presenters for permission to use the images.
More details and the available slide decks are here, Twitter hashtag - #RuleofFive2019.
Call for Open Source Antibiotics Fragment Contributions
What we have: Fragment hits from an initial screen against MurE and MurD, performed at Diamond screening facility, and a platform to screen additional fragment libraries or follow-up compounds.
What we need: Additional chemical matter for screening. The Diamond screening platform is high-throughput and we would ideally be able to take full advantage of this.
Full details are on the Open Source Antibiotics website

Covalent Modification
I've written a page in the Drug Discovery Resources on covalent inhibitors.
This publication suggests this area is going to become more important "An activity-guided map of electrophile-cysteine interactions in primary human immune cells" https://www.biorxiv.org/content/10.1101/808113v1.
Here, we present a global map of cysteines in primary human T cells that are susceptible to covalent modification by electrophilic small molecules. More than 3000 covalently liganded cysteines were found on functionally and structurally diverse proteins, including many that play fundamental roles in immunology.
European Lead Factory
Great news! European Lead Factory has restarted.
Pivot Park Screening Centre has successfully completed the first ultra-High Throughput Screening in IMI’s ESCulab project, and as such restarting the operations of the European Lead Factory. The screening on the European Compound Collection of ~500.000 compounds using a biochemical 1536-wells assay was finished within 4 days. Currently triaging of the UK owned program is ongoing within the Consortium, applying further biochemical and biophysical follow-up assays as well as the resynthesis of promising hits.
The programme is currently accepting proposals http://www.europeanleadfactory.eu/drug-target-assays.
First call for proposals to the Psychiatry Consortium
A new initiative, great to see efforts in CNS diseases.
First call for proposals to the Psychiatry Consortium. Read more about the kind of projects the Psychiatry Consortium is looking to fund on the website https://md.catapult.org.uk/syndicates/psychiatry-consortium/?.
History of rare diseases and their genetic causes - a data driven approach
One of the advantages of being a consultant is that I can feel free to contribute to projects that I find interesting. So as well as working with a couple of Open-Source drug discovery projects (e.g. Open Source Antibiotics I can also follow a couple of rare disease programs.
This publication looks very useful History of rare diseases and their genetic causes - a data driven approach.
This dataset provides information about monogenic, rare diseases with a known genetic cause supplemented with manually extracted provenance of both the disease and the discovery of the underlying genetic cause of the disease.
More details of how the dataset was constructed.
We collected 4166 rare monogenic diseases according to their OMIM identifier, linked them to 3163 causative genes which are annotated with Ensembl identifiers and HGNC symbols. The PubMed identifier of the scientific publication, which for the first time describes the rare disease, and the publication which found the gene causing this disease were added using information from OMIM, Wikipedia, Google Scholar, Whonamedit, and PubMed. The data is available as a spreadsheet and as RDF in a semantic model modified from DisGeNET.
A very interesting read.
Open Targets Updated
The latest Open Targets Platform 19.09 has been released. The latest release contains
27,024 targets 10,474 diseases 3.33 million pieces of evidence 7.78 million associations between targets and diseases
In addition, a number of Target Enabling Packages (TEP) provided by Structural Genomics Consortium have been included, there are more details here. Several new chemical probes have also been included.
Covalent Inhibtors page updated
I've updated the covalent inhibitors page to include details of two covalent fragment libraries that have been used in fragment screening.

In Silico Toxicology' Network Meeting 2019
A new initiative that should be very useful,
An event (with free registration) to foster the In silico Toxicology Community in the UK and beyond. Scientific contributions are as welcome as those on ongoing work, regulatory aspects, industry perspectives, databases, relevant software, journals etc. in the field. This event is meant to stimulate interactions and discussions, hence speakers are asked to present both about in silico toxicology approaches that are already useful to be applied at the current stage, as well as aspects that don't work that well right now, and where future developments are hence needed.
Venue 26 November 2019, Unilever Lecture Theatre, Department of Chemistry, University of Cambridge (CB2 1EW)
Full details and link for registration here In Silico Toxicology' Network Meeting 2019.
Ester and Amide bioisosteres page updated
A small update to the Ester and Amide bioisosteres page.
Drug Discovery Resources
Just checking the half-year viewing stats for the Drug Discovery Resources section of the website.
The Drug Discovery Resources pages are intended to act as a resource for scientists undertaking drug discovery, they were initially based on a course I give but have been expanded to give much more detail and to cover subjects not covered in the course.
Over the first six months there were nearly 35,000 users with users on average viewing 2-3 pages. The visitors come from 152 different countries with the US (33%), UK (14%) , India (8%), Germany (3.2%) and Canada (3.1%) topping the list.
The most viewed pages over this period were
- Lipophilicity
- Calculating Physicochemical Properties
- Distribution and Plasma Protein Binding
- Bioisosteres
- Molecular Interactions
- Kinase Inhibitors
- Acid Bioisosteres
- Aromatic Bioisosteres
- Solvation and desolvation
- Aspartic Acid Proteases
- CYP Interactions
- Fragment based screening
- HERG
Looking at the operating systems 56% are Windows users, 20% Mac users, 10% iOS and 10% Android, Chrome dominates the browser stats (65%) with Safari second (17%) and Firefox third (10%).
Open-access Antimicrobial Screening Database
I just got news of the first public release of CO-ADD screening data
CO-ADD is a non-for-profit initiative led by academics at The University of Queensland. Our goal is to screen compound for antimicrobial activity for academic research groups and generate a public knowledge database for the development of novel agents for the treatment of microbial infections. The knowledge base contains chemical structures and antimicrobial activity data from CO-ADD’s screening, made publicly available by the academic research groups, with more data to be released over time.
The database is available here.

More on hydrogen bonding interactions
Studies of hydrogen bond strength and directionality are largely based on crystal structures which can be biased by crystal packing forces. In a systematic QM study of a wide range of hydrogen bonding groups by Diogo Santos-Martins, Stefano Forli, 34 donors and 67 acceptors DOI they showed there is no correlation between the strength of the hydrogen bond and directionality.
Results demonstrate that there are very strong HB acceptors (e.g.,DMSO) with nearly isotropic interactions, and weak ones (e.g.,thioacetone) with a sharp directional profile. Similarly, groups can have comparable directional propensity, but be very distant in the strength spectrum (e.g., thioacetone and pyridine).
This work also includes a fabulous sheet in the supplementary information giving details of interaction energies for various groups.
I've updated the molecular interactions page.
Funding Opportunities
The GHIT Fund announces an investment opportunity for the Target Research Platform
The GHIT Fund announces an investment opportunity for the Target Research Platform (TRP) in Partnership with the Wellcome Trust. The TRP investments are intentionally broad in potential scope and focus on new technologies and novel approaches.
TRP investments are intentionally broad in potential scope and focus on new technologies and novel approaches. Proposals must be within the project scope and investment eligibility below in order to be considered. The TRP is currently focused on technologies and approaches that address unmet or priority needs within malaria, tuberculosis, HIV/AIDS and Neglected Tropical Diseases listed in the GHIT Intent to Apply form.
MRC/AstraZeneca Centre for Lead Discovery
The MRC/AstraZeneca Centre for Lead Discovery (CLD) aims to support academic researchers in discovering potential starting points for small molecule therapeutic approaches with a clear line-of-sight to therapeutic use. The MRC/AZ Centre for Lead Discovery will form a unique cornerstone for academic and industrial drug discovery projects by supplying high throughput screening (HTS) infrastructure (NiCoLA-B).
Canada-UK Artificial Intelligence Initiative
The call aims to support innovative and cutting-edge interdisciplinary AI research that encourages the exploration of new interdisciplinary research methodologies, approaches and tools that cuts across at least two of the following research domains:
- social sciences and humanities;
- health and biomedical sciences; and
- natural sciences and engineering (including computational and/or mathematical sciences).
There is also a more comprehensive listing of grant funding here.
Where Drugs Come From: A Comprehensive Look
I was going to highlight this article a while back "Academia and industry: allocating credit for discovery and development of new therapies" DOI but got distracted. However I notice that Derek Lowe has written a commentary that is far more detailed than I could have written on his In the Pipeline Blog.
You can read it here Where Drugs Come From it is well worth spending a little reading, digesting and then sending the link to others.
canSAR BLACK
cansar black v1.1.1 now available - includes improved search, new protein family page, and performance improvements
canSAR is an integrated knowledge-base that brings together multidisciplinary data across biology, chemistry, pharmacology, structural biology, cellular networks and clinical annotations, and applies machine learning approaches to provide drug-discovery useful predictions. canSAR’s goal is to enable cancer translational research and drug discovery through providing this knowledge to researchers from across different disciplines. It provides a single information portal to answer complex multi-disciplinary questions including - among many others: what is known about a protein, in which cancers is it expressed or mutated and what chemical tools and cell line models can be used to experimentally probe its activity? What is known about a drug, its cellular sensitivity profile and what proteins is it known to bind that may explain unusual bioactivity?
Open Targets Platform: release 19.06 is out
The latest update of the Open Targets Platform, release 19.06 is available.
This update includes
Target safety information
As a follow-up to the safety data in Open Targets Platform release 19.04, now has more targets with known safety effects and safety risk information, including TBXA2R and JAK2.
TEPs and chemical probes
In this release, they've included the latest Target Enabling Packages (TEPs) for GALT, GALK1 and MLLT1. Also added more chemical probes, small-molecule modulators of a protein’s function that can be used in cell-based or animal studies.
Target-disease associations
A new release always means new evidence available for novel target-disease associations.
Early career MedChem workshop
The Early Career MedChem Workshop is a pre-meeting workshop taking place on the Sunday afternoon before the 20th SCI/RSC Medicinal Chemistry Symposium, Churchill College, Cambridge, Sunday 8 - Wednesday 11 September 2019.
It is aimed at early career (up to 5 years’ experience) medicinal chemists. Registration for this event will be at no additional cost to the main meeting.
The workshop will consist of a team-based exercise around a virtual medicinal chemistry programme and will offer participants:
- Training in advanced medicinal chemistry
- Testing out leadership/decision making skills
- Networking between potential future leaders of different organisations
- Introductions of possible future collaborators from pharma, CRO, charity and SME sectors
- Opportunity for informal interaction with experienced medicinal chemists workshop facilitators who will be open to questions around their own experiences
- Attendance at the full 20th SCI/RSC Medicinal Chemistry Symposium including lectures on a full range of drug targets, key enabling processes and technologies
Feedback from previous events has been excellent for this unique learning experience.
Also note there are student bursaries to help fund attendance at this event, email conferences@soci.org for more information.
Upcoming Conferences
I just thought I'd mention a couple of meetings I'm helping to organise.
2nd RSC-BMCS / RSC-CICAG Artificial Intelligence in Chemistry
Artificial Intelligence is presently experiencing a renaissance in development of new methods and practical applications to ongoing challenges in Chemistry. Following the success of the inaugural “Artificial Intelligence in Chemistry” meeting in 2018 a second meeting has been organised at Fitzwilliam College, Cambridge (2nd to 3rd September 2019). The lineup is now finalised and looks like a great selection of speakers. There is still time to submit posters (closing date 5th July).

Registration is open and there are discounts for RSC members.
The Twitter hashtag - #AIChem19 is already being actively used.
20th SCI/RSC Medicinal Chemistry Symposium
This is Europe’s premier biennial Medicinal Chemistry event, focussing on first disclosures and new strategies in Medicinal Chemistry. It takes place a Churchill College, Cambridge UK, 8 September - 11 September 2019. There is a fantastic lineup of speakers and looks to be one of the highlights of the MedChem calendar. Early career scientists can also take part in a Medicinal chemistry workshop on the Sunday afternoon, a great way for people to learn medicinal chemistry and meet other scientists in a fun and informal setting.
You can register here both RSC and SCI members get a reduced rate, and despite the slightly confusing page on the SCI website you don't have to be a member to attend, just select "Event Member FREE from the dropdown menu and you can register for the event without membership.

Twenty Years of the Rule of Five
It has been over twenty years since Lipinski published his work determining the properties of drug molecules associated with good solubility and permeability. Since then, there have been a number of additions and expansions to these “rules”. There has also been keen interest in the application of these guidelines in the drug discovery process and how these apply to new emerging chemical structures such as macrocycles.
This meeting aims to have a look at the impact the Ro5 has had on drug discovery and as well as looking to the future and how we use these rules in the changing drug compound landscape as drug discovery moves into novel areas of chemistry.

There is a very exciting group of speakers and the timetable has been designed to allow a panel discussion after each session. Given the topic and the speakers I'm sure these will be entertaining sessions.
You can register here and there are discounts for RSC members
Twitter hashtag - #RuleofFive2019
European Lead Factory looking for novel screening programmes again
The European Lead Factory has been funded for another round of screening activities but under a new name European Screening Centre: unique library for attractive biology ESCulab. Over the next five years, the European Lead Factory will initiate 185 new drug discovery projects by screening medically relevant drug targets from European researchers, small and medium-sized enterprises and pharmaceutical industry against the ELF library of 550,000 unique chemical compounds.
The European Lead Factory was launched in 2013 and set up a joint collection of half a million compounds and a state-of-the-art high throughput screening centre. By the time the project ended last year, they had delivered results to researchers in universities, small biotechs and large companies across Europe, helping them to identify potential new drug candidates and breathing new life into a range of disease areas. In many cases, the seeds sown by the European Lead Factory resulted in new patents, partnering deals, and two start-ups. Now, a new IMI project, ESCulab will build on the work of the European Lead Factory. This means that researchers with drug targets can apply to screen the project’s compound collection for hits and get help developing any compounds further if they like. Jon de Vlieger, coordinator of the ESCulab consortium at Lygature, said: ‘It’s truly exciting to continue the onboarding of new and innovative proposals for screening and provide high quality starting points for drug discovery to academics and SMEs throughout Europe. In an effort to broaden our scope we are not only looking for target-based approaches, but now also enable phenotypic screens.’
You can apply here. If you don't have an assay in a format suitable for ultra high-throughput screening it is worth noting that the Wellcome Trust have small awards designed to help with the technology change required for HTS.
Medicines Used
We often see news stories about the "biggest" drugs based on sales, however this way of looking at drug sales is somewhat skewed by the high cost of some therapeutics, particularly biologics. It is also noteworthy that the majority of the drugs are indicated for cancer.
| Drug | Indication | Worldwide Sales 2018 |
|---|---|---|
| Humira | Rheumatoid Arthritis | $19.936 billion |
| Eliquis | Anticoagulant | $9.872 billion |
| Revlimid | Cancer | $9.685 billion |
| Opdivo | Cancer | $7.570 billion |
| Keytruda | Cancer | $7.171 billion |
| Enbrel | Rheumatoid Arthritis | $7.126 billion |
| Herceptin | Cancer | $6.981 billion |
| Avastin | Cancer | $6.847 billion |
| Rituxan | Cancer | $6.750 billion |
| Xarelto | Anticoagulant | 6.589 billion |
| Eylea | Cancer/AMD | $6.551 billion |
| Remicade | Crohn's Disease | $5.908 billion |
| Prevnar 13 | Pneumonia | $5.802 billion |
| Stelara | Psoriasis | $5.156 billion |
| Lyrica | Epilepsy/Pain | $4.970 billion |
I can't help but think that a better metric might be the number of patients treated. Whilst I don't have access to worldwide prescriptions the NHS in the UK does provide some information as part of the Prescription cost analysis for 2018. Whilst the number of prescriptions does correspond exactly with the number of patients treated I suspect it gives a very good indication.
Looking at the categories of drugs it is interesting to note that cancer does not figure in the top 20 categories. As you might expect lipid-lowering drugs, gastric ulcer treatment, treatments for cardiovascular disease, anti-depressants and analgesics are the most prescribed.
| Drug Category | Number of items |
|---|---|
| Lipid-Regulating Drugs | 74,289,246 |
| Proton Pump Inhibitors | 60,024,837 |
| Angiotensin-Converting Enzyme Inhibitors | 44,159,042 |
| Calcium-Channel Blockers | 41,102,081 |
| Beta-Adrenoceptor Blocking Drugs | 38,617,728 |
| Selective Serotonin Re-Uptake Inhibitors | 38,216,924 |
| Non-Opioid Analgesics And Compound Prep | 35,998,332 |
| Antiplatelet Drugs | 34,139,843 |
| Thyroid Hormones | 32,253,535 |
| Control Of Epilepsy | 27,989,893 |
| Vitamin D | 24,559,380 |
| Opioid Analgesics | 23,393,193 |
| Selective Beta(2)-Agonists | 22,900,805 |
| Biguanides | 21,806,787 |
| Corticosteroids (Respiratory) | 20,879,110 |
| Angiotensin-II Receptor Antagonists | 20,499,156 |
| Oral Anticoagulants | 17,613,257 |
| Tricyclic & Related Antidepressant Drugs | 16,704,980 |
| Other Antidepressant Drugs | 15,911,182 |
| Thiazides And Related Diuretics | 14,628,130 |
| Antihistamines | 13,626,254 |
Looking at the top most prescribed drugs, small molecule drugs dominate for all indications.
| Chemical Name | Indication | Items |
|---|---|---|
| Atorvastatin | Lipid-Regulating Drugs | 41,820,664 |
| Levothyroxine Sodium | Thyroid Hormones | 32,187,950 |
| Omeprazole | Proton Pump Inhibitors | 31,038,076 |
| Amlodipine | Calcium-Channel Blockers | 29,052,338 |
| Ramipril | Angiotensin-Converting Enzyme Inhibitors | 28,605,025 |
| Lansoprazole | Proton Pump Inhibitors | 25,461,167 |
| Simvastatin | Lipid-Regulating Drugs | 24,303,261 |
| Bisoprolol Fumarate | Beta-Adrenoceptor Blocking Drugs | 23,625,562 |
| Colecalciferol | Vitamin D | 23,609,903 |
| Aspirin | Non-Opioid Analgesics And Compound Prep | 23,397,042 |
| Metformin Hydrochloride | Diabetes | 21,806,787 |
| Salbutamol | Selective Beta(2)-Agonists | 21,577,054 |
| Paracetamol | Non-Opioid Analgesics And Compound Prep | 18,516,491 |
| Co-Codamol (Codeine Phos/Paracetamol) | Opioid Analgesics | 15,179,951 |
| Sertraline Hydrochloride | Selective Serotonin Re-Uptake Inhibitors | 14,815,719 |
| Citalopram Hydrobromide | Selective Serotonin Re-Uptake Inhibitors | 14,136,645 |
| Amitriptyline Hydrochloride | Tricyclic & Related Antidepressant Drugs | 13,532,567 |
| Furosemide | Thiazides And Related Diuretics | 11,945,445 |
| Beclometasone Dipropionate | Corticosteroids (Respiratory) | 10,671,698 |
Also of note is the number of prescriptions for drugs that are readily available over the counter.
No Support for Historical Candidate Gene or Candidate Gene-by-Interaction Hypotheses for Major Depression Across Multiple Large Samples
For depression, SLC6A4 seemed like a great candidate and was supported by very early gene studies
BUT….
Am J Psychiatry. 2019 May 1;176(5):376-387. DOI
The study results do not support previous depression candidate gene findings, in which large genetic effects are frequently reported in samples orders of magnitude smaller than those examined here. Instead, the results suggest that early hypotheses about depression candidate genes were incorrect and that the large number of associations reported in the depression candidate gene literature are likely to be false positives.
How many other early gene disease association studies need to be checked?
Updated Drug Discovery Resources
I've done some updates to the Drug Discovery Resources.
The Following Pages have been Updated
Macrocycles
Predicting Metabolism
Covalent Inhibitors
PROteolysis TArgeting Chimeras (PROTACs), Lysosome Targeting Chimeras (LYTACs), and ENDosome TArgeting Chimeras (ENDTACs)
Twenty Years of the Rule of Five
RSC-BMCS and RSC-CICAG are delighted to announce registration is now open for Twenty Years of the Rule of Five, Wednesday, 20th November 2019, Sygnature Discovery, BioCity, Nottingham, UK. #RuleofFive2019
It has been over twenty years since Lipinski published his work determining the properties of drug molecules associated with good solubility and permeability. Since then, there have been a number of additions and expansions to these “rules”. There has also been keen interest in the application of these guidelines in the drug discovery process and how these apply to new emerging chemical structures such as macrocycles.

This symposium will bring together researchers from a number of different areas of drug discovery and will provide a historical overview of the use of Lipinski’s rules, as well as looking to the future and how we use these rules in the changing drug compound landscape.
Full details and registration are here https://www.maggichurchouseevents.co.uk/bmcs/twenty-years-Ro5.htm.
Cathepsin C inhibitor chemical probe
As part of the Boehringer Ingelheim's efforts to foster innovation, they are share selected molecules with the scientific community all for free. The opnme portal gives access to a range of novel ligands. The latest addition is BI-9740
BI-9740 is a very potent and highly selective inhibitor of the enzymatic activity of Cathepsin C. It blocks human CatC in vitro with an IC50 of 1.8 nM and shows > 1500x selectivity versus the related proteases Cathepsin B, F, H, K, L and S. BI-9740 displays no activity against 34 unrelated proteases from different classes up to a concentration of 10 µM.

Chemical probes are absolutely essential for target validation and it is great to see so many high quality tools being made available.
European Lead Factory update
The European Lead Factory (ELF) secured a total project budget of EUR 36.5 million under the second framework of the Innovative Medicines Initiative (IMI). 20 partners in 7 countries will push forward the transformation of potential drug targets to new medicines in the new project ESCulab (European Screening Centre: unique library for attractive biology) under the European Lead Factory brand.
Full details here https://www.europeanleadfactory.eu/news-events/european-lead-factory-europe’s-largest-collaborative-drug-discovery-platform-continues.
Over the next five years, the European Lead Factory will initiate 185 new drug discovery projects by screening medically relevant drug targets from European researchers, small and medium-sized enterprises and pharmaceutical industry against the ELF library of 550,000 unique chemical compounds.
European universities dismal at reporting results of clinical trials
Clinical trial data is some of the most important information in Drug Discovery, after all it is humans we are looking to treat! However analysis of 30 leading institutions found that just 17% of study results had been posted online. The 30 universities surveyed are those that sponsor the most clinical trials in the EU. Fourteen of these institutions had failed to publish a single results summary.

The Universities that have failed to publish a single trial result are highlighted in red in the table below.

The contrast between the UK universities and the rest of Europe could not be starker,
UK universities in the survey performed significantly better than those in the rest of Europe. The University of Oxford and King’s College London had both published 93% of the trial results due on the register, and University College London had posted 81%.
According to the report every single medical university in the UK is currently working hard to upload missing clinical trial results onto the EU registry, and in many cases onto other registries such as ISRCTN and the US registry Clinicaltrials.gov as well. This demonstrates that where there is a will, there is a way.
If we remove the UK Universities from the analysis the level of reporting falls to a pitiful 7%.
Lack of transparency in clinical trials harms patients. The timely posting of summary results is an ethical and scientific obligation.
2nd RSC-BMCS / RSC-CICAG Artificial Intelligence in Chemistry
The lineup for the 2nd RSC-BMCS / RSC-CICAG Artificial Intelligence in Chemistry Monday-Tuesday, 2nd to 3rd September 2019 Fitzwilliam College, Cambridge, UK has been updated.

Twitter #AIChem19
Artificial Intelligence is presently experiencing a renaissance in development of new methods and practical applications to ongoing challenges in Chemistry. Following the success of the inaugural “Artificial Intelligence in Chemistry” meeting in 2018, we are pleased to announce that the Biological & Medicinal Chemistry Sector (BMCS) and Chemical Information & Computer Applications Group (CICAG) of the Royal Society of Chemistry are once again organising a conference to present the current efforts in applying these new methods. The meeting will be held over two days and will combine aspects of artificial intelligence and deep machine learning methods to applications in chemistry.
Speakers
Deep learning applied to ligand-based de novo design: a real-life lead optimization case study, Quentin Perron, IKTOS, USA
A Turing test for molecular generators, Jacob Bush, GlaxoSmithKline, UK
Presentation title to be confirmed, Keynote: Regina Barzilay, Massachusetts Institute of Technology, USA
Artificial intelligence for predicting molecular Electrostatic Potentials (ESPs): a step towards developing ESP-guided knowledge-based scoring functions, Prakash Rathi, Astex Pharmaceuticals, UK
Molecular transformer for chemical reaction prediction and uncertainty estimation, Alpha Lee, University of Cambridge, UK
Drug discovery disrupted - quantum physics meets machine learning, Noor Shaker, GTN, UK
Presentation title to be confirmed, Christian Tyrchan, AstraZeneca,
Presentation title to be confirmed, Anthony Nicholls, OpenEye Scientific Software, USA
Deep generative models for 3D compound design from fragment screens, Fergus Imrie, University of Oxford, UK
DeeplyTough: learning to structurally compare protein binding sites, Joshua Meyers, BenevolentAI, UK
Presentation title to be confirmed, Maciej Haranczyk, IMDEA, Spain
Deep learning for drug discovery, Keynote: David Koes, University of Pittsburgh, USA
Presentation title to be confirmed, Olexandr Isayev, University of North Carolina at Chapel Hill, USA
Dreaming functional molecules with generative ML models, Christoph Kreisbeck, Kebotix, USA
Presentation title to be confirmed, Keynote: Adrian Roitberg, University of Florida, USA
Applications for poster presentations are welcomed, the closing date for submission is 5th July. A number of RSC-BMCS and RSC-CICAG student bursaries are available up to a value of £250, to support registration, travel and accommodation costs for PhD and post-doctoral applicants studying at European academic institutions. The closing date for bursary applications is 15th July.
Full details are on the conference website
Atomwise AIMS awards

I suspect many will have noticed the recent announcement of the Early Results in Drug Discovery Partnership with AI Biotech Company. These are the first results of the Atomwise AIMS awards:
The researchers have been using Atomwise’s AI-powered in silico screening technology to develop therapeutic treatments for, among others, certain types of strokes, hand-foot-and-mouth disease, and an infection that causes reproductive failure in pigs.
The AIMS award program is a great opportunity for university research scientists to easily access AI-assisted structure-based virtual screening technology:
- Customized small molecule virtual screen using AtomNet™ technology
- 72 small molecules predicted to bind to a specific target protein – QC verified by mass spectrophotometry, resuspended and diluted to a convenient concentration, aliquoted into microtiter plates, and delivered at no cost to the researcher
- Support from Atomwise’s medicinal chemists and structural biologists
- Opportunity to receive up to $30K USD to subsidize assay work
If you have a target protein with an X-ray crystal, Cryo-EM, or NMR structure, or with close sequence homology to a protein with available structures, and an assay in place to evaluate 72 potential hits, then you should consider applying.
Full details are on the AIMs awards page and the closing date is 29 April 2019.
OpenSource Antibiotics
I just thought I’d highlight a new project I’m involved with.
Open Source Antibiotics (https://github.com/opensourceantibiotics) is intended to be a platform for a collaborative effort towards antibiotic discovery.

The first projects have been initiated
Mur Ligase (https://github.com/opensourceantibiotics/murligase) and the background to these exciting targets can be found on the wiki page.
https://github.com/opensourceantibiotics/murligase/wiki
This also provides details of the first two fragment screens.
MurD https://github.com/opensourceantibiotics/murligase/wiki/MurD-fragment-screen
and
MurE https://github.com/opensourceantibiotics/murligase/wiki/MurE-fragment-screen
What we want now is for people to join in and suggest the next round of fragments that should be screened. Ideally these should be commercially available but if people want to design, make and submit their own fragments we would be happy to screen them.
If you feel appropriate, we would appreciate any publicity on this exciting new project
Early Career MedChem Workshop
One of the highlights of the SCI/RSC Cambridge MedChem Meetings the Early Career MedChem Workshop, a satellite workshop consisting of a team-based exercise around a virtual medicinal chemistry programme will be held on the Sunday afternoon prior to this event, aimed at early career (up to 5 years’ experience) medicinal chemists. Registration for this event will be at no additional cost to the main meeting, Read the flyer here.

Registration is on the 20th SCI / RSC Medicinal Chemistry Symposium website
Lysosome Targeting Chimeras (LYTACs)
A while back I added a page on PROteolysis Targeting Chimera (PROTAC) a technology using the ubiquitin proteasome system to induce degradation of the target protein DOI. However this technology is limited to cytosolic proteins.
A recent publication highlights a new technology "Lysosome Targeting Chimeras (LYTACs) for the Degradation of Secreted and Membrane Proteins" DOI that further extends the protein degradation options.
Targeted protein degradation is a powerful strategy to address the canonically undruggable proteome. However, current technologies are limited to targets with cytosolically-accessible and ligandable domains. Here, we designed and synthesized conjugates capable of binding both a cell surface lysosome targeting receptor and the extracellular domain of a target protein…. LYTACs represent a modular strategy for directing secreted and membrane proteins for degradation in the context of both basic research and therapy.
The past, present and future of anti-malarial medicines
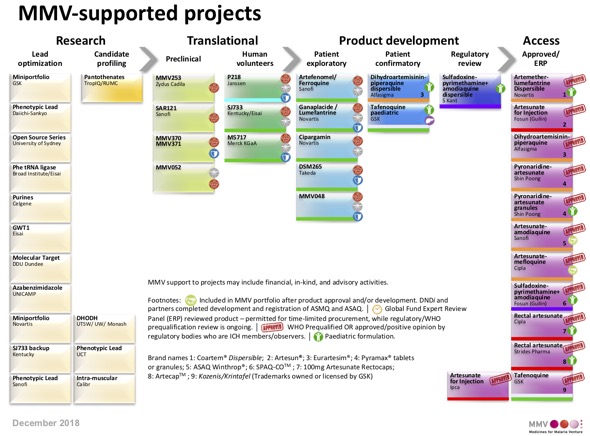
A fantastic Open Access review of Malaria Drug Discovery DOI. Covers the history of Malaria medicines going back to quinine through to the latest MMV supported projects and our current understanding of some of the emerging novel molecular targets.
20th Cambridge MedChem meeting Registration open
I know the is eagerly anticipated because I have folder of email enquiries I can now respond to.
Registration for the 20th Cambridge MedChem Meeting is now open !!
https://www.soci.org/events/20th-scirsc-medicinal-chemistry-symposium.
Really is a fabulous lineup of speakers. Also includes Malcolm Campbell Memorial Prize presentation
20th Cambridge MedChem meeting
On 8-11 September 2019 we will be holding the 20th Cambridge Medicinal Chemistry Symposium, this marks a significant milestone for this meeting and the committee would like to use the opportunity to highlight some of the first disclosures of now important medicines and other events from the 40 year history of this meeting.
We have all the programmes from the 13th to 19th meetings, and I've managed to get details of the first meeting shown in this PDF. If you could share scanned copies of programme from the 2nd to 12th meetings it would be really great.
Also if you have particular memories of meetings you would be willing to share feel free to send an email.

Website Update
I've spent some time over the last couple of weeks updating and adding new content to the Drug Discovery Resources section of the website.
In particular, s drug targets become more challenging medicinal chemists are looking at alternatives to small molecule competitive inhibitors, the section on covalent inhibitors have been expanded and a new page on PROTACs has been added. PROTACs are bifunctional molecules that bind to the target protein and an E3 ligase, the simultaneous PROTAC binding of two proteins brings the target protein in close enough proximity for polyubiquitination by the E2 enzyme associated to the E3 ligase, which flags the target protein for degradation through the proteasome. This offers a powerful alternative to competitive inhibition.

The Probes & Drugs portal has been added to the chemical probes page, this is a public resource joining together focused libraries of bioactive compounds (probes, drugs, specific inhibitor sets etc.) with commercially available screening libraries.
The page describing commercial fragment screening libraries has been updated to include a couple of new additions and flagging some that seem to be unavailable, if I've missed any feel free to let me know.
The section on hERG has been updated with links to new references and details of hERGcentral.
hERGCentral: A Large Database to Store, Retrieve, and Analyze Compound-Human Ether-à-go-go Related Gene Channel Interactions to Facilitate Cardiotoxicity Assessment in Drug Development. The hERGCentral database hergcentral.org is based on experimental data obtained from a primary screen by electrophysiology against more than 300,000 structurally diverse compounds screened at 1 and 10uM.
Unfortunately the database appears to be no longer available. Whilst the supplementary information for the original publication does not contain the structures of the tested compounds it does reference the PubChem substance ID. I used these identifiers to download the structures of the >300,000 records and combined them with the experimental data provided in the Excel tables in the supplementary information. The complete dataset can be downloaded from the hERG page.
Small molecules can potentially bind to a variety of bimolecular targets and whilst counter-screening against a wide variety of targets is feasible it can be rather expensive and probably only realistic for when a compound has been identified as of particular interest. For this reason there is considerable interest in building computational models to predict potential interactions the page on predicting bioactivities has been expanded.
The section on bioisosteres also have a few new examples.
hERG central data
A publication by Fang et al DOI describes hERGCentral: A Large Database to Store, Retrieve, and Analyze Compound-Human Ether-à-go-go Related Gene Channel Interactions to Facilitate Cardiotoxicity Assessment in Drug Development. The hERGCentral database hergcentral.org is based on experimental data obtained from a primary screen by electrophysiology against more than 300,000 structurally diverse compounds screened at 1 and 10uM. Unfortunately the database appears to be no longer available. Whilst the supplementary information for the original publication does not contain the structures of the tested compounds it does reference the PubChem substance ID. I used these identifiers to download the structures of the >300,000 records and combined them with the experimental data provided in the Excel tables in the supplementary information. The complete dataset can be downloaded here in either
or in
Bear in mind this is single point data and there will be a fair amount of scatter.

I've added this information to the page on hERG.
RSC Medicinal Chemistry Residential School 2019
The Royal Society of Chemistry Medicinal Chemistry Residential School takes place 2 - 7 June 2019, Loughborough, United Kingdom is a fantastic opportunity for anyone starting out or contemplating a career in Drug Discovery.
The school is designed for graduate and post-doctoral chemists with 1-5 years’ experience in the field of drug research. Drug discovery is an interdisciplinary subject so delegates from biological or computational backgrounds will benefit from attendance at the school. In addition, final year PhD students from pharmaceutical or organic chemistry contemplating a career in drug discovery are also encouraged to attend.
The course includes the following topics:
- Target Validation
- Computational Chemistry
- Biological Mechanisms
- Pharmacokinetics and Drug Metabolism
- Screening of New Compounds
- Patents
- Molecular Biology in Medicinal Chemistry
- Exploiting a Chemical Lead
- Combinatorial Chemistry and Molecular Diversity
- Case Histories of Drug Discovery
- Toxicology in Drug Discovery
- Pharmaceutical Considerations in Drug Development
- Structure-guided Drug Design
- Physical Properties and Quantitative Structure-Activity Relationships
- Hints and Tips in Medicinal Chemistry
Full details here http://www.rsc.org/events/detail/33379/medicinal-chemistry-residential-school-2019.
Promises, promises, and precision medicine
A very interesting commentary on the impact (or lack of) genomics has had on human healthcare. J Clin Invest. 2019
The promises of precision medicine are to dramatically change patient care via individually tailored therapies and, as a result, to prevent disease, improve survival, and extend healthspan.
However, nearly two decades after the first predictions of dramatic success, we find no impact of the human genome project on the population’s life expectancy or any other public health measure, notwithstanding the vast resources that have been directed at genomics. Exaggerated expectations of how large an impact on disease would be found for genes have been paralleled by unrealistic timelines for success, yet the promotion of precision medicine continues unabated.
In light of the limitations of the precision medicine narrative, it is urgent that the biomedical research community reconsider its ongoing obsession with the human genome and reassess its research priorities including funding to more closely align with the health needs of our nation. We do not lack for pressing public health problems. We must counter the toll of obesity, inactivity, and diabetes; we need to address the mental health problems that lead to distress and violence; we cannot stand by while a terrible opiate epidemic ravages our country; we have to prepare conscientiously for the next influenza pandemic; we have a responsibility to prevent the ongoing contamination of our air, food, and water. Topics such as these have taken a back seat to the investment of the NIH and of many research universities in a human genome–driven research agenda that has done little to solve these problems, but has offered us promises and more promises.
The human genome project was undoubtedly a magnificent achievement, but has the investment in genomics delivered?
There is an extended discussion on In the Pipeline https://blogs.sciencemag.org/pipeline/archives/2019/01/31/precision-medicine-real-soon-now.
Ten threats to global health in 2019
A recent publication by the World Health Organisation makes sobering reading.
The world is facing multiple health challenges. These range from outbreaks of vaccine-preventable diseases like measles and diphtheria, increasing reports of drug-resistant pathogens, growing rates of obesity and physical inactivity to the health impacts of environmental pollution and climate change and multiple humanitarian crises.
Here are 10 of the many issues that will demand attention from WHO and health partners in 2019.
- Air pollution and climate change
- Noncommunicable diseases (diabetes, cancer and heart disease)
- Global influenza pandemic
- Fragile and vulnerable settings (combination of drought, famine, conflict, and population displacement)
- Antimicrobial resistance
- Ebola and other high-threat pathogens
- Weak primary health care
- Vaccine hesitancy (the reluctance or refusal to vaccinate despite the availability of vaccine)
- Dengue
- HIV
Medicines for Malaria Venture call for proposals
MMV has announced a call for drug discovery proposals
1. Compounds addressing the key priorities of the malaria eradication agenda
Novel families of molecules in the hit-to-lead or lead optimization stages are sought without G6PD deficiency liabilities that either:
Kill or reactivate hypnozoites for use as part of a P. vivax radical cure; or have activity against sexual stage V gametocytes and evidence of transmission blocking in SMFA.
2. Compounds having activity against asexual liver and/or blood stages
Novel chemical series with EC50<500nM and which have one or more of the following key features:
A known, novel mechanism of action; An inability to select resistant mutants in vitro; Activity at more than one life-cycle stage; A long half-life (ideally >4h in rodents) and confirmed in vivo efficacy. For advanced series, we are seeking novel compounds with, ideally, a predicted human half-life >100h and a predicted oral single human dose <500mg or an i.m. dose that can be administered in <1mL and sufficient for up to 3 months’ protection in humans.
3. Novel approaches for screening
To help identify new phenotypic and/ or target based hits, as well as confirm activity of MMV compounds on all human malaria asexual blood stages, new screening proposals are sought amongst the three categories below:
Validated Plasmodium target-based assays, ideally with evidence of target essentiality beyond asexual blood stages. Biological validation should be supported by a biological target based screening assay suited for identification of novel chemical series. Novel whole cell phenotypic screening paradigms to potentially identify new relevant chemistry. Asexual blood stage assays for vivax and ovale malaria.
Compounds for Target Identification
MMV also welcomes requests for support to investigate the mechanism of action of compounds:
Call for African proposals
Finally, MMV welcomes proposals from endemic region African scientists focused in the following priority areas:
Compounds with confirmed activity on any antimalarial life-cycle stage. Novel families of molecules with confirmed activity (EC50 < 10uM) and a medicinal chemistry plan that tackles any known or anticipated liability. Priority will be given to proposals that maximize use of local natural products.
Assay development and screening
Interesting meetings
In many companies/institutions/universities new arrivals are presented with a variety of desktop tools with little or no advice on how to use them other than "pick it up as you along". This workshop is intended to provide expert tutorials to get you started and show what can be achieved with the software.
The tutorials will be given a series of outstanding experts Christian Lemmen (BioSolveIT), Akos Tarcsay (ChemAxon), Giovanna Tedesco (Cresset), Dan Ormsby (Dotmatics) Greg Landrum (Knime ) and Matt Segall (Optibrium), you will be able to install the software packages on you own laptops together with a license to allow you to use it for a limited period after the event.
Registration opened just before Christmas and apparently there were a number of people sign up over the festive period. Remember there are a limited number of places and it is first come first served.
Registration and full details are here.

Also a free one-day symposium Streamlining Drug Discovery" in Frankfurt
The very successful symposia series "Streamlining Drug Discovery" comes to Frankfurt on 14 February 2019. Jointly BioSolveIT, Optibrium, Lhasa and Elsevier invite you for this free one-day event highlighting new approaches and technologies being applied to the search for future therapeutics. For further details please visit the symposium website https://www.biosolveit.de/symposium/2019-02-14/
How Structural Biologists and the Protein Data Bank Contributed to Recent FDA New Drug Approvals
An interesting review DOI
Discovery and development of 210 new molecular entities (NMEs; new drugs) approved by the US Food and Drug Administration 2010–2016 was facilitated by 3D structural information generated by structural biologists worldwide and distributed on an open-access basis by the PDB. The molecular targets for 94% of these NMEs are known. The PDB archive contains 5,914 structures containing one of the known targets and/or a new drug, providing structural coverage for 88% of the recently approved NMEs across all therapeutic areas. More than half of the 5,914 structures were published and made available by the PDB at no charge, with no restrictions on usage >10 years before drug approval. Citation analyses revealed that these 5,914 PDB structures significantly affected the very large body of publicly funded research reported in publications on the NME targets that motivated biopharmaceutical company investment in discovery and development programs that produced the NMEs.
Annual Website Review
As 2019 starts I'd like to wish you all a Happy New Year and hope for great success in your drug discovery endeavours.
The Drug Discovery Resources website continues to increase in popularity with over 147,000 page views, an increase of 8% over the figure for 2017. The pages were visited by over 72,500 viewers and around a third of the visitors come back on multiple occasions suggesting they find it useful. The visitors come from 172 different countries with the US (32%), UK (13%) and India (8%) topping the list.
The most viewed pages in 2018 were
- Lipophilicity
- Distribution and Plasma Protein Binding
- Bioisosteres
- Calculating Physicochemical Properties
- Molecular Interactions
- Kinase Inhibitors
- Aspartic Acid Protease Inhibitors
- Solvation and desolvation
- Hit Identification
- CYP Interactions
- Acid Bioisosteres
- Fragment based screening
- HERG
There have been a number of significant updates to the Drug Discovery Resources this year, in particular, a new section on Macrocycles which has proved very popular. The Target Validation section has been updated several times, as has the Molecular Interactions page and I'm grateful for readers who have pointed out relevant recent publications.

Interestingly the Books and Grant funding research have seasonal peaks in viewing.
Looking at the operating systems 56% are Windows users, 20% Mac users, 10% iOS and 10% Android, Chrome dominates the browser stats (64%) with Safari second (17%) and Firefox third (10%).