Urea replacements

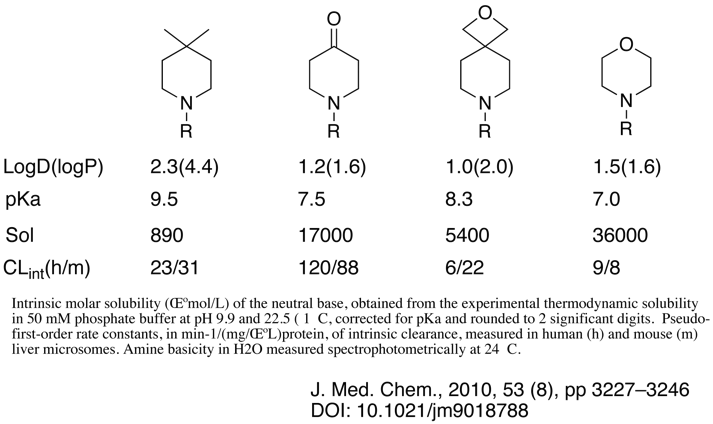
An interesting paper by Wuitschik et al looks at oxetanes as replacements for carbonyl and how they might influence physicochemical properties, whilst a variety of different structures are compared I’ve just pulled out the data for 4-substituted piperidines. Whilst the influence on pKa is intermediate between piperidine and the corresponding 4-piperidone the more polar oxetane means that the resulting LogD is actually lower. This could be useful if trying to reduce HERG, of CYP activity.
Last Update 12 November 2011
Simple Bioisosteres
Carbonyl Bioisosteres
Back to Drug Discovery Resources
