Serine Protease Inhibitors
Serine proteases are proteases in which one of the conserved amino acids in the active site of the enzyme is serine. Three major classes have been defined based on the physicochemical properties of the P1 site, trypsin-like (positively charged residues Lys/Arg preferred at P1), elastase-like (small hydrophobic residues Ala, Val at P1) or chymotrypsin-like (large hydrophobic residues Phe/Tyr/Leu at P1). The serine proteases are characterized by a catalytic triad of residues (Ser195, His57 and Asp102, chymotrypsin numbering system) that is responsible for amide bond hydrolysis.
The catalytic mechanism is shown below, it is thought to involve the catalytic triad Asp, His and Ser, abstraction of the proton from the Ser transforms the hydroxyl from a poor nucleophile into an excellent one. Subsequent nucleophilic attack on the carbonyl of the substrate amide by the serine results in the formation of a tetrahedral intermediate that is stabilised by hydrogen bonding of the incipient anion to backbone amides (oxyanion hole). His-57 then transfers a hydrogen to the nitrogen of the amide bond resulting in an acylated serine and departure of the amine. The acylated serine is then hydrolysed by water catalysed by the His, Asp to regenerate the enzyme.
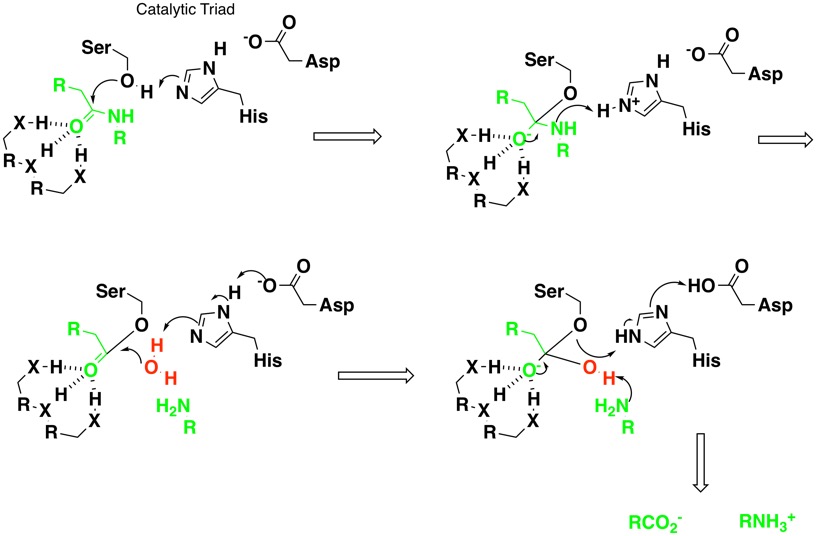
For more information on the mechanism have a look at the MACiE database (Mechanism, Annotation and Classification in Enzymes), Trypsin, PDB entry 1PQ5 is an example of a serine protease.
Inhibitors
A number of inhibitors have sought to exploit the nucleophilic serine on example is the use of an electron-deficient carbonyl such as an trifluroketone, the crystal structure (1GG6 ) of chymotrypsin with a trifluroketone inhibitor is shown below with the catalytic triad highlighted in red.
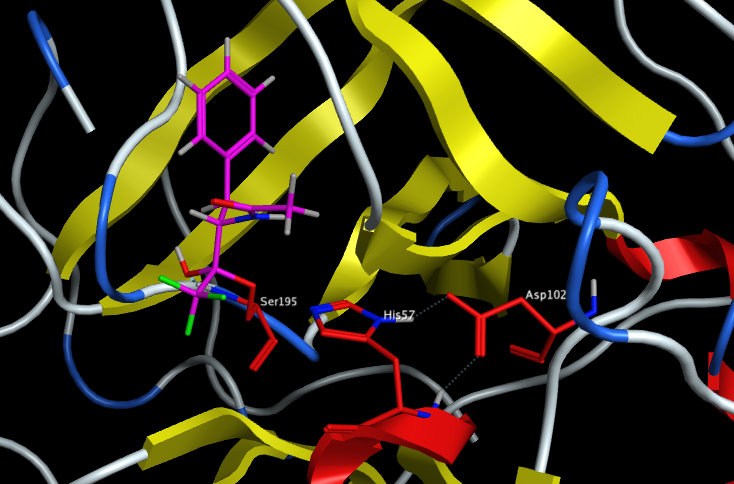
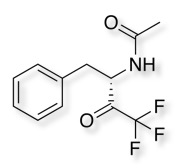
The nucleophilic serine attacks the electron-deficient ketone to form a stablised tetrahedral complex.
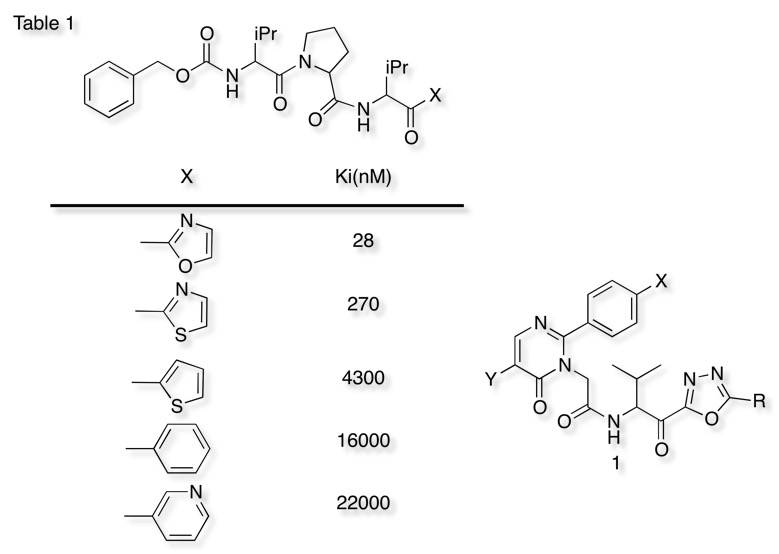
A number of other inhibitors have been identified that exploit the nucleophilic serine, either forming stable acyl intermediates (sulphonyl flourides/chlorides, phosphonates), or stable tetrahedral intermediates (aldehydes, halomethyl ketones, boronic acids.) More recently a number of alpha-keto heterocycles have been explored as agents that would enhance the susceptibility of the carbonyl towards nucleophilic attack by the serine. Edwards et al have shown that elastase can be inhibited by alpha ketohetrocycles (Table 1) and that the more electron-withdrawing heterocycles are the most potent inhibitors. The was subsequently supported by QM/MM calculations. This approach has also included oxadiazoles 1.
Worth reading Strategies for the inhibiton of serine proteases CMLS, Cell. Mol. Life Sci. 58 (2001) 596‚ Inhibitors of proteases and amide hydrolases that employ an α-ketoheterocycle as a key enabling functionality Bioorganic & Medicinal Chemistry Volume 16, Issue 4, 15 February 2008, Pages 1562-1595
Updated 5 March 2012