ADME Properties
Optimization of the ADME (Absorption, Distribution, Metabolism, and Excretion) properties of the drug molecule is often the most difficult and challenging part of the whole drug discovery process. The ADME profile will also have a major impact on the likelihood of success of a drug.
Physicochemical Properties and ADME
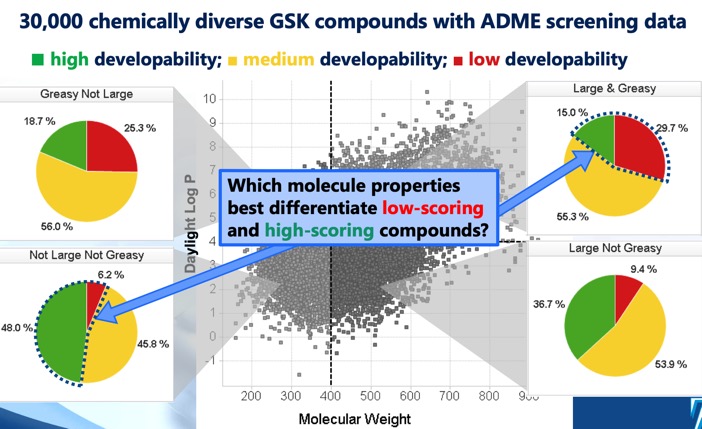
The Developability score DOI identifies four distinct cLog P/molecular weight regions that define optimal and sub-optimal chemical space, and a developability score derived from regression models using solubility, permeability, protein binding and 3A4 inhibition screening data. Whilst the sector MWt <400, cLogP <4 suggested the greatest chance of success, it was noted that even the MWt >400, cLogP >4 sector included some developable molecules albeit at a much lower chance of success. Much of this was discussed at the 20 years Rule of Five meeting and the meeting report is available here
See also the section on Formulation, Solubility
Hints that all is not well with the pharmacokinetic profile of a drug candidate.
- Dose proportionality issues, increasing dose does not lead to a corresponding increase in plasma levels :- Absorption issues
- Good plasma levels but little in the brain:- Active efflux (PGP substrate).
- Plasma levels decrease on repeat dosing:- Enzyme induction
- Plasma levels increase when co-administered with another drug:- Enzyme inhibition.
- Duration of action does not match half-life :-Active metabolites or slow dissociation from the target.
A General Strategy for Integrating ADME in Drug Discovery
Keeping an eye of molecular properties throughout the drug discovery process is a critical aspect of the process, the focus at different stages is tabulated below.
- Hit Identification, In silico design of screening libraries (physicochemical properties, flag compounds using ADME models), experimental measurement of logP/D and solubility if possible.
- Lead Identification, 96 (or 384) well assays for cell penetration (PAMPA, MDCK-hMDR), microsomal stability, CYP3A inhibition (the most common CYP involved in drug metabolism), rat pharmacokinetics. LogP pKa and solubility measured.
- Lead Optimisation, 96 (or 384) well assays for transporters (if cell and/or brain penetration is an issue), CYP inhibition for multiple isoforms, CYP induction, plasma protein binding, plasma/microsomal/hepatocyte stability in multiple species (human, species used for efficacy studies, species to be used for safety). Metabolite identification. In vivo PK study in species used in efficacy studies to determine PK/PD relationships. In vivo PK study in species to be used in safety studies.
- Candidate Identification, multiple dose stars in safety species, prediction of human PK and potential for drug drug interactions. Think about formulations, especially for likely top doses in safety. Maximum tolerated dose studies. See also preclinical checklist.
See Sections on
- Absorption Assays that can be used to evaluate absorption properties, interpretation and possible strategies to provide solutions.
- Distribution Assays and properties of molecules effecting plasma protein binding.
- Brain Penetration Assays and properties of molecules influencing brain penetration
- Transporters proteins involved in transporting molecules/ions into and out of cells
- Metabolism Assays, interpretation of in vitro data, General Strategies for reducing metabolism.
- Excretion (In Progress)
I’ve added an analysis of the CYP inhibition in ChEMBL
PKPD simulation
XenologiQ have produced a PKPD simulation tool that is described and available for download here This is a tool to analyse in vitro & in vivo ADME & PK data. Predictions for human PK & dose can be made based on available data & assumptions about efficacious concentration. Guidance is given on how secure such projections are likely to be given the nature and congruence of the data available. The training materials include theoretical background, slides, video presentations & test data for hands-on exercises.
Clinical ADME Studies
The FDA have published guidelines for Clinical Drug Interaction Studies this guidance helps sponsors of investigational new drug applications and applicants of new drug applications evaluate drug-drug interactions (DDIs) during drug development and communicate the results and recommendations from DDI studies. these can be supported by in vitro studies In Vitro Metabolism-and Transporter Mediated Drug-Drug Interaction Studies, this guidance is intended to help drug developers plan and evaluate studies to determine the drug-drug interaction (DDI) potential of an investigational drug product.
Worth Reading
Increasing small molecule drug developability in sub-optimal chemical space, Med. Chem. Commun., 2013,4, 673-680, DOI
In most drug discovery projects sorting out the ADME/T issues is the most challenging part of the project. Handbook of Essential Pharmacokinetics, Pharmacodynamics and Drug Metabolism for Industrial Scientists provides a useful desktop reference for PK/PD issues and can provide answers to questions like "What is the plasma volume of a Guinea Pig?".
If you want an authorative book on the molecular properties of drug like molecules then Drug-like Properties: Concepts, Structure Design and Methods: From ADME to Toxicity Optimization is the book for you.
Last Updated 25 November 2019