Bioisosteric Replacements
Carbonyl Replacements
Urea replacements

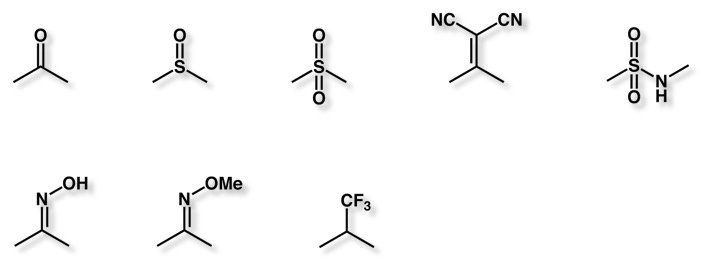
Ketone replacements
An interesting paper by Wuitschik et al looks at oxetanes as replacements for carbonyl and how they might influence physicochemical properties, whilst a variety of different structures are compared I’ve just pulled out the data for 4-substituted piperidines. Whilst the influence on pKa is intermediate between piperidine and the corresponding 4-piperidone the more polar oxetane means that the resulting LogD is actually lower. This could be useful if trying to reduce HERG, of CYP activity.

Intrinsic molar solubility (Eºmol/L) of the neutral base, obtained from the experimental thermodynamic solubility in 50 mM phosphate buffer at pH 9.9 and 22.5 ( 1 C, corrected for pKa and rounded to 2 significant digits. Pseudo-first-order rate constants, in min-1/(mg/EºL)protein, of intrinsic clearance, measured in human (h) and mouse (m) liver microsomes. Amine basicity in H2O measured spectrophotometrically at 24 C.
J. Med. Chem., 2010, 53 (8), pp 3227–3246 DOI
3-Hydroxyoxetanes have also been investigated as carboxylic acid bioisosteres.
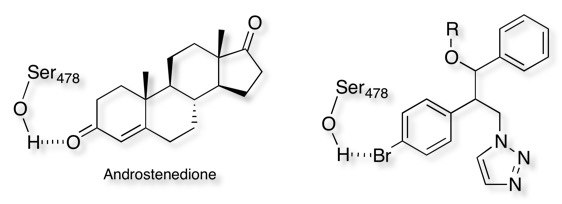
In the X-ray structure of aromatase Ser478 is part of a water-mediated network of hydrogen bonding, enabling the interaction between aromatase and the C3-keto oxygen of androstenedione, McNulty et al DOI were able to use an aryl halide as a bioisosteric mimic for the cyclohexenone.
Last Update 23 July 2017