Bioisosteric Replacements
Ring Replacements
There are a variety of rings found in drugs an analysis of drugs listed in the FDA Orange Book identified 351 ring systems, the m10 most common are shown below DOI.

Alkyl rings are often major sites for metabolism, and one of the best strategies for reducing metabolism is the introduction of heteroatoms. This is described in detail in the work by Stepan et al in their work on γ-Secretase Inhibitors. The starting point was the cyclohexyl analog of the sulphonamide shown below.
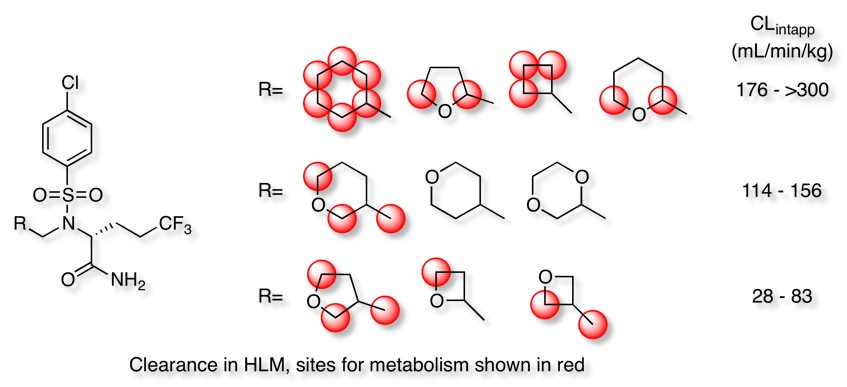
As shown below introduction of heteroatoms and reducing the ring size serve to reduce LogP and reduce the intrinsic clearance in HLM. It seems that the more solvent exposed oxygen of the 3- or 4-substituted ethers has a more pronounced effect.
The piperidine ring is the third most often found ring in drug discovery DOI and there have been some interesting efforts to identify bioisosteric replacements. Often the key sites of metabolism are the atoms adjacent to the piperidine nitrogen this can sometimes be reduced by increasing the polarity of the ring by replacement by morpholine (although this will also influence the pKa). Alternatively, this can also be influenced by substitution by changing to the tropane system. More recently spirosystems have been identified as possible bioisosteric replacements DOI. Substitution on the Azaspiro[3.3]heptane ring system can also explore vectors not accessible from the piperidine ring.

Piperazines are the second most popular ring system, offering easy derivatization and favourable physicochemical properties. Many sample collections contain many, many structures containing the Piperazine motif the bioisosteres described below offer the opportunity to modify the pKa of the nitrogens but also subtly alter the directionality of the vectors from the nitrogens. DOI.

4-(Pyrimidin-4-yl)morpholines are privileged pharmacophores for PI3K and PIKKs inhibition by virtue of the morpholine oxygen hydrogen bonding to the hinge Val882.

As shown below the 4-(Pyrimidin-4-yl)morpholine (orange) adopts a conformation where the morpholine is almost coplanar with the pyrimidine ring. Replacement of the morpholine ring by pyran (purple) results in the pyran ring twisting orthogonal to the pyrimidine ring. In contrast, the cyclopropyl pyran (cyan) adopts a conformation similar to the morpholine CCDC 1864315.


See also Aromatic Bioisosteres.
Worth reading
Rings in Drugs DOI.
Last Update 17 Feb 2024