Privileged Structures
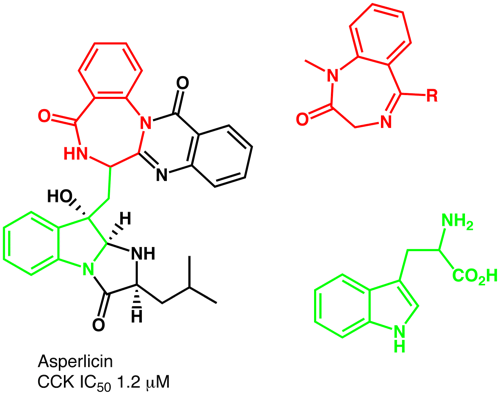
The term "privileged structures" was first coined by Ben Evans DOI: 10.1021/jm00120a002 who recognised the potential of certain regularly occuring structural motifs as templates for derivatization to discovery novel ligands for binding to proteins. In this seminal paper they identified a benzodiazepine and substitued indole as key structures in their work to yield CCK antagonists.
More recently Klaus Mueller has tried to define the term privileged structure more specifically. "Small, non-planar structures with robust conformations that provide interesting 3D exit vectors for substitution, with drug-like properties and ideally readily accessible synthetically." The structure often consists of a semi-rigid scaffold which is able to present multiple hydrophobic residues without undergoing hydrophobic collapse. In particular he has used the benzodiazepine framework to illustrate many examples in which the 7-membered ring can be locked in specific chair-like or boat-like conformations with the appropriate substitution.
Whilst many templates could be used in perhaps an additional limitation is that it has been suggested that whilst a medium sized protein may have 10-20 bonding pockets, ligands typically access pockets 5 angstroms apart.

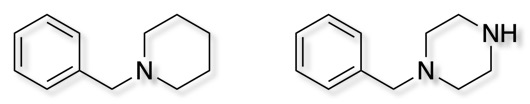
Two very popular privileged structures are N-benzyl piperidine and N-benzyl piperazine. They offer a variety of different interactions (pi-stacking, hydrophobic, electrostatic) with a relatively well defined 3D structures.
A recent publication gives a very nice summary of their use in drug discovery DOI.
Abstract The N-benzyl piperidine (N-BP) structural motif is commonly employed in drug discovery due to its structural flexibility and three-dimensional nature. Medicinal chemists frequently utilize the N-BP motif as a versatile tool to fine-tune both efficacy and physicochemical properties in drug development. It provides crucial cation-π interactions with the target protein and also serves as a platform for optimizing stereochemical aspects of potency and toxicity. This motif is found in numerous approved drugs and clinical/preclinical candidates. This review focuses on the applications of the N-BP motif in drug discovery campaigns, emphasizing its role in imparting medicinally relevant properties. We provide an overview of approved drugs, the clinical and preclinical pipeline, and discuss its utility for specific therapeutic targets and indications, along with potential challenges.
In addition there are certain substructures confer potency within a class of targets based on a key molecular interaction. For example hydroxamates confer potency for matrix metalloproteases (thiols for zinc), benzamidine for serine proteases, heterocycles co-ordinating to the haem in cytochrome P450s, and aminopyrimidines for kinases and ATP binding proteins.
Many of these frameworks have been used as templates for the design of libraries of molecules for drug discovery DOI 10.1016/j.cbpa.2010.02.018
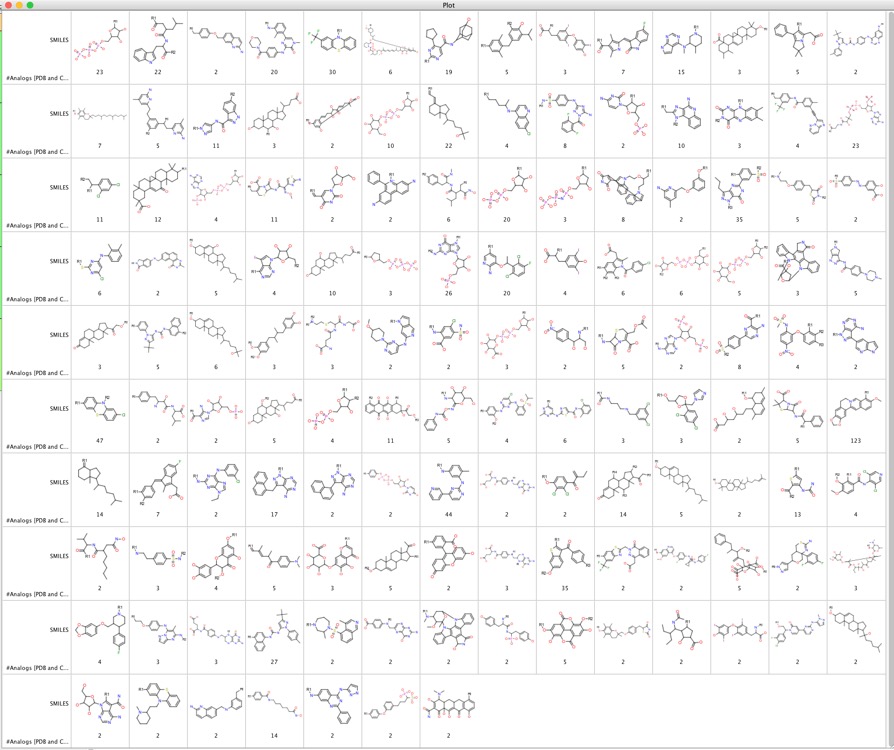
A recent publication describes a related approach looking for multi target ligands, a systematic analysis of currently available X-ray structures for compounds forming complexes with different targets DOI, by using X-ray structures they aim to avoid molecules that might interfere with the assay, some of these ligands were described in the medicinal chemistry literature, making it possible to consider additional target annotations and search for analogues. This work identified 133 unique analogue-series-based scaffolds were isolated that can serve as templates for the design of new compounds with multitarget activity are shown below (click on image to enlarge).
Updated 17 Jul 2024