Lipophilicity is possibly the lost important physicochemical property of a potential drug, it plays a role in solubility, absorption, membrane penetration, plasma protein binding, distribution, CNS penetration and partitioning into other tissues or organs such as the liver and has an impact on the routes of clearance. It is important in ligand recognition, not only to the target protein but also CYP450 interactions, HERG binding, and PXR mediated enzyme induction.
LogP is a component of Lipinski’s Rule of 5 a rule of thumb to predict drug-likeness.
The most commonly used measure of lipophilicity is LogP, this is the partition coefficient of a molecule between an aqueous and lipophilic phases, usually octanol and water.
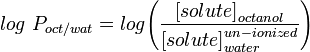
Measurement of LogP can be undertaken in a variety of ways, the most common is the shake-flask method, which consists of dissolving some of the solute in question in a volume of octanol and water, shaking for a period of time, then measuring the concentration of the solute in each solvent. This can be time-consuming particularly if there is now quick spectroscopic method to measure the concentration of the molecule in the phases. A faster method of log P determination makes use of high-performance liquid chromatography. The log P of a solute can be determined by correlating its retention time with similar compounds with known log P value.
Calculation of lipophilicity
Usually it is not practical to experimentally determine the LogP of every compound made (and it may be of interest to calculate logP prior to synthesis) and so calculated results are used, there are a number of software tools available both desktop and online (don’t use for confidential compounds).
Many of these applications work by using a large training data-set of known values to determine fragment contributions for sub-structures and functional groups, however logP is not a simple additive property and correction terms are needed to allow for proximity effects, H-bonding, electronic effects etc. as shown in the examples below.

For unknown functional groups the programs often approximate using individual atom contributions. Because the training sets and the algorithms vary between applications it is very important not to combine calculated results using different tools.
Some of the tools allow the user to extend the training set using in house measured values, this may be critical when exploring novel functional groups.
LogD
However the majority of known drugs contain ionisable groups and are likely to be charged at physiological pH and LogP only correctly describes the partition coefficient of neutral (uncharged) molecules. LogD the distribution constant is a better descriptor of the lipophilicity of a molecule. This can be determined in a similar manner to LogP but instead of using water, the aqueous phase is adjusted to a specific pH using a buffer. Log D is thus pH dependent, hence the one must specify the pH at which the log D was measured. Of particular interest is the log D at pH = 7.4 (the physiological pH of blood serum).
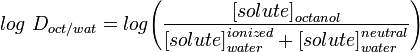
Applications like Marvin allow the user to calculate the logD but also display the pH distribution profile, as shown below for Warfarin.

For compounds with a pKa close to physiological pH it may be critical to consider what might actually be the predominant ionised form.

This can also be valuable when thinking about absorption from the different regions of the alimentary canal where the pH ranges from 1-3 in the stomach to 7-8 in the ileum.
Updated 14 April 2010
Back to Physiochemical Properties
Back to Drug Discovery Resources
