pKa
The behaviour of all drugs is dependent on their physicochemical properties and since a significant proportion of drugs contain ionisable centers a knowledge of their pKa is essential.
The pKa is defined as the negative log of the dissociation constant
Where the dissociation constant is defined thus:-
Most drugs have pKa in the range 0-12, and whilst it is possible to calculate pKa it is desirable to experimentally measure the value for representative examples. There are a number of instruments that are capable of measuring pKa or LogP/D an example being the Sirius T3

Usually it is not practical to experimentally determine the pKa of every compound made and so calculated results are used, there are a number of software tools available both desktop and online (don’t use for confidential compounds). In the image below I show a scatter plot created using Vortex of experimental versus calculated pKa values determined using the ChemAxon tools. I used the command line version
cxcalc -i ID -o /Users/swain/Desktop/pka2.sdf '/Users/swain/Desktop/pka_data.sdf' pka -a 3 -b 3
To calculate the top three acid and basic ionisations for a file containing the structures of over 1000 compounds for which I had culled experimental pKa from the literature. The structures ranged from small molecules containing 4-8 heavy atoms to over 300 drug molecules approved by the FDA. The process took only a few seconds and the results are shown below. In general the results are pretty good and probably within the variation seen for experimentally determined data seen in the literature. There are some outliers and I plan to look at using extra experimental data to retrain the algorithm.
Applications like Marvin allow the user to calculate the pKa but also display the pH distribution profile, this can be valuable when thinking about absorption from the different regions of the alimentary canal where the pH ranges from 1-3 in the stomach to 7-8 in the ileum.
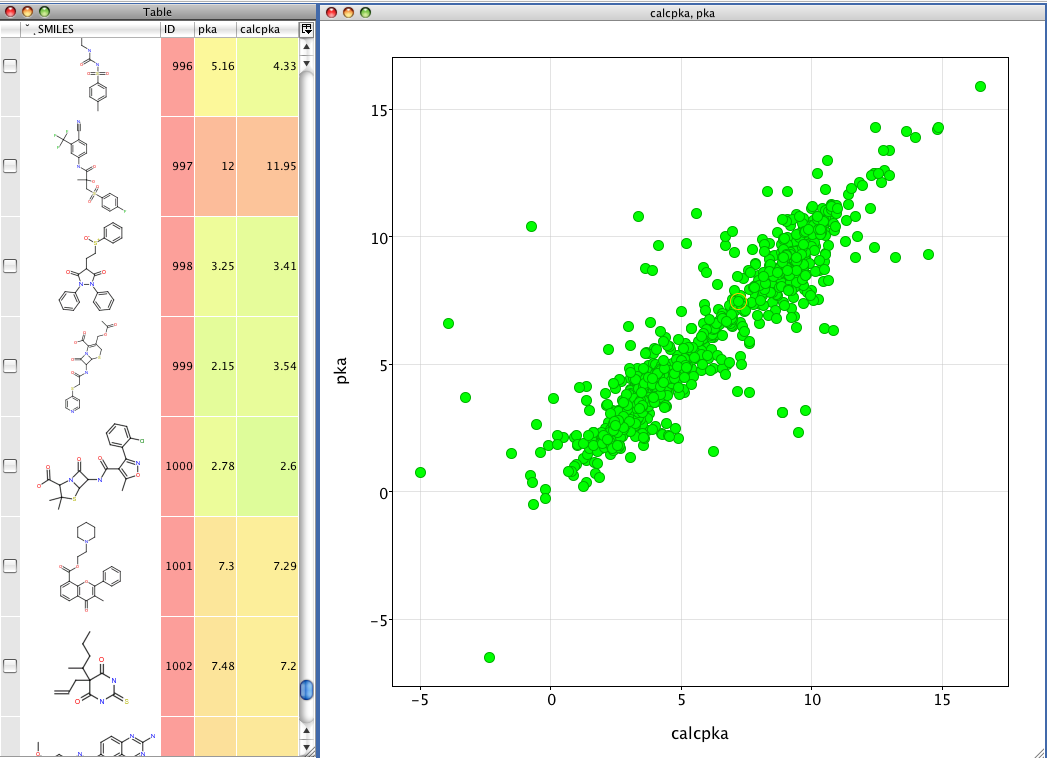
First looking at a weak acid like Warfarin
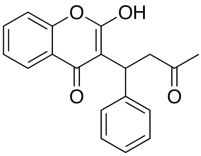
Ionisation of Warfarin, at low pH the neutral species predominates (orange line) at high pH it is the ionised species (green line).

Proportion of ionised/unionised species at different points in the alimentary canal.
| Location/pH | Stomach/pH=2 | Duodenum/pH=6 | Jejunum/pH=7.5 | Blood/pH=7.4 |
| Unionized (%) | 100 | 88 | 18 | 25 |
| Ionized(%) | 0 | 12 | 82 | 75 |
The reverse is seen with a weakly basic drug such as Arecoline, at low pH the ionised species predominates (orange line) at high pH it is the neutral species (green line).

Ionisation of Arecoline
Proportion of ionised/unionised species at different points in the alimentary canal.
| Location/pH | Stomach/pH=2 | Duodenum/pH=6 | Jejunum/pH=7.5 | Blood/pH=7.4 |
| Unionized (%) | 0 | 2 | 37 | 36 |
| Ionized(%) | 100 | 98 | 63 | 64 |
Theoretically, weakly acidic drugs (eg, aspirin) are more readily absorbed from an acid medium (stomach) than are weakly basic drugs (eg, quinidin ). However, whether a drug is acidic or basic, much of the absorption can occur in the small intestine because the surface area is larger and membranes are more permeable.
Updated 14 April 2010
See also Tuning Basicity
Back to Physiochemical Properties
Back to Drug Discovery Resources
