The term "privileged structures" was first coined by Ben Evans1 (Merck) who recognised the potential of certain regularly occuring structural motifs as templates for derivatization to discovery novel ligands for binding to proteins. In this seminal paper they identified a benzodiazepine and substitued indole as key structures in their work to yield CCK anatagonists.

More recently Klaus Mueller has tried to define the term privileged structure more specifically. "Small, non-planer structures with robust conformations that provide interesting 3D exit vectors for substitution, with drug-like properties and ideally readily accessible synthetically." The structure often consists of a semi-rigid scaffold which is able to present multiple hydrophobic residues without undergoing hydrophobic collapse. Whilst many templates could be used in perhaps an additional limitation is that it has been suggested that whilst a medium sized protein may have 10-20 bonding pockets, ligands typically access pockets 5 angstroms apart.
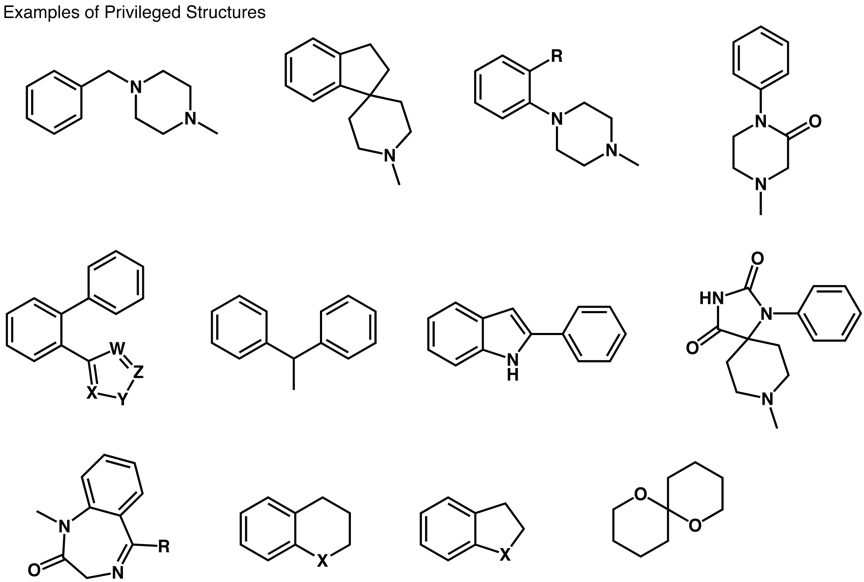
In addition there are certain substructures confer potency within a class of targets based on a key molecular interaction. For example hydroxamates confer potency for matrix metalloproteases (thiols for zinc), benzamidine for serine proteases, heterocycles co-ordinating to the haem in cytochrome P450s, and aminopyrimidines for kinases and ATP binding proteins.
1. Evans, B. E.,et al. (1988) J. Med. Chem. 31, 2235-2246.
Last Updated 26 March 2010
See also Fragment Collection
See also Sample Collection
Back to Drug Discovery Resources
