Aspartic proteases are a family of protease enzymes that use two highly conserved aspartic acid residues in the active site for catalytic cleavage of their peptide substrates. Perhaps the most extensively studies as drug discovery targets are Rennin (Chymosin), beta-secratase, the Plasmempsins for the treatment of malaria, and HIV protease. HIV protease is unusual in that it is a homodimer and each of the monomeric units contribute an aspartic acid.
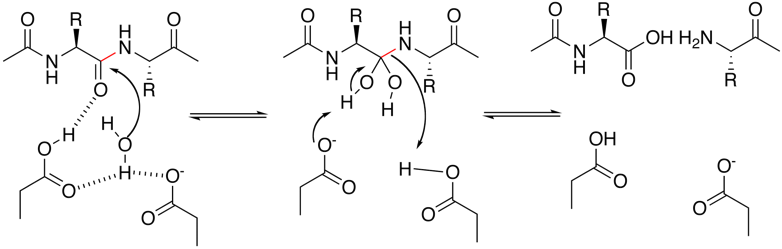
The generally accepted mechanism of action is a general acid-base mechanism involving coordination of a water molecule between the two highly conserved aspartate residues.One aspartate activates the water by abstracting a proton, enabling the water to attack the carbonyl carbon of the substrate scissile bond, generating a tetrahedral oxyanion intermediate. Rearrangement of this intermediate leads to protonation of the scissile amide.
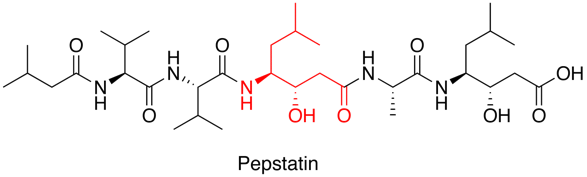
Nearly all known aspartyl proteases are inhibited by pepstatin a naturally occurring hexa-peptide containing the unusual amino acid statine (Sta, (3S,4S)-4-amino-3-hydroxy-6-methylheptanoic acid).
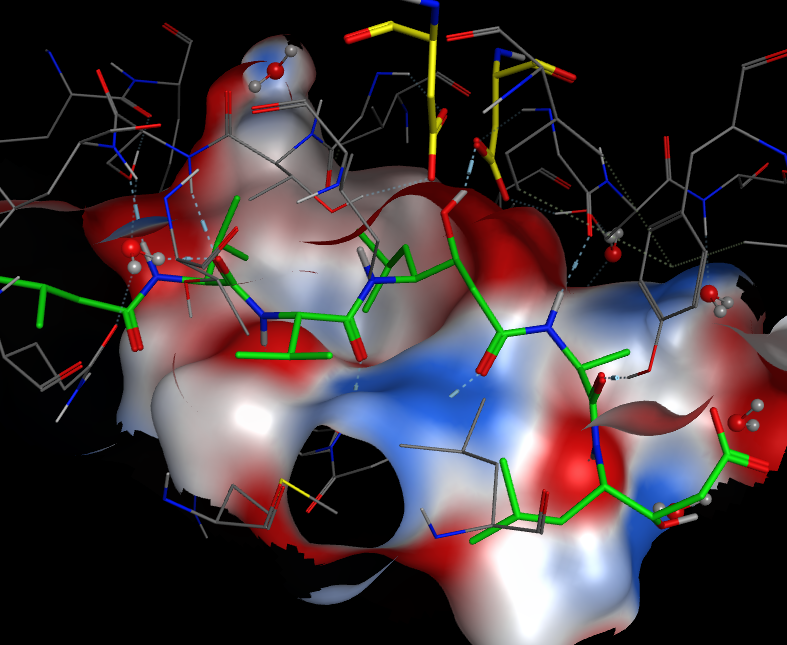
The image below shows pepstatin (green) bound into the ative site of an aspartic protease, the hydroxyl group can be seen hydrogen bonding to one of the aspartic acids (yellow) mimicking the tetrahedral transition state of the reaction. The protein surface is coloured by charge (red positive, blue negative).
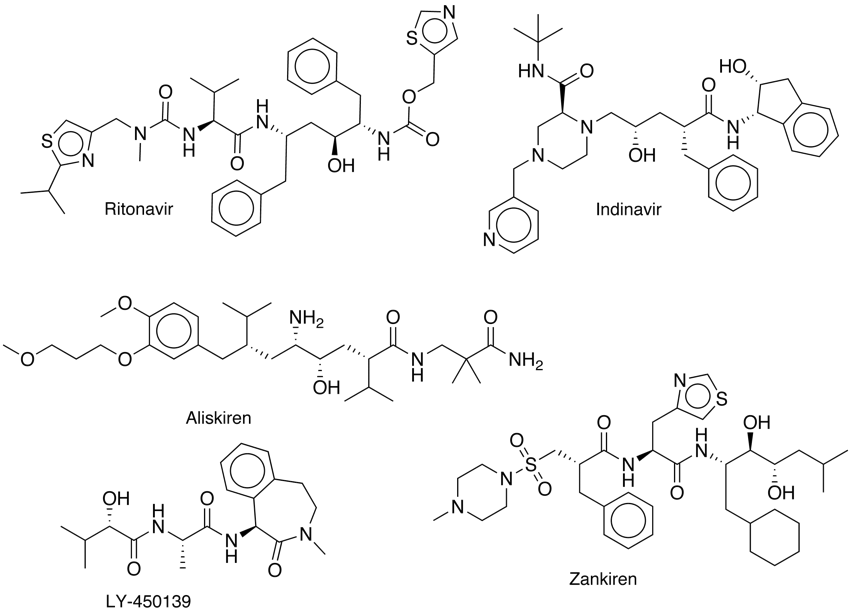
The statin motif has been used to design a range of inhibitors based on the idea of a non-hydrolysable mimic of the transition state formed during amide hydrolysis.
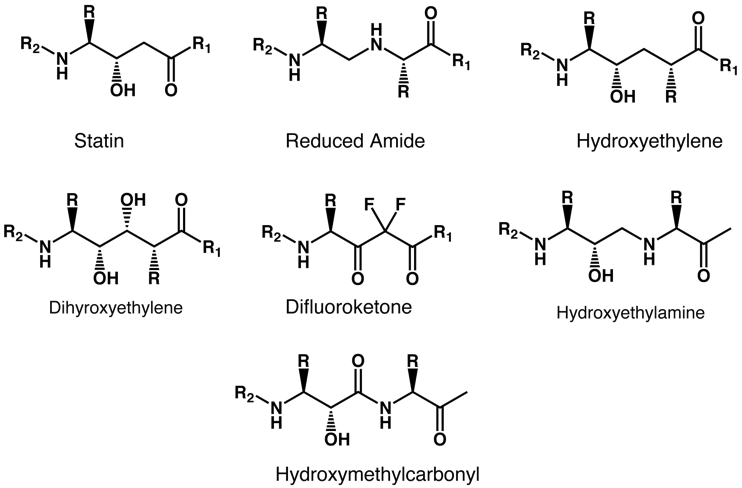
A number of these ideas have been developed into potent inhibitors, these include Ritonavir and Indinavir inhibitors of HIV-1 protease, the rennin inhibitors Zankiren and Aliskiren and the BACE inhibitor LY-450139.
See Also
Kinase Inhibitors
Cysteine Protease Inhibitors
Serine Protease Inhibitors
Metalloprotease Inhibitors
Back to Drug Discovery Resources
Updated 8 May 2011
