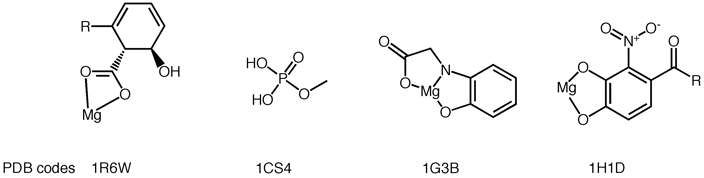
A hard ion like magnesium is almost always octahedrally coordinated predominantly to oxygen.
If you see an octahedrally coordinated 'water' in a protein crystal structure, you can bet it's really a magnesium.
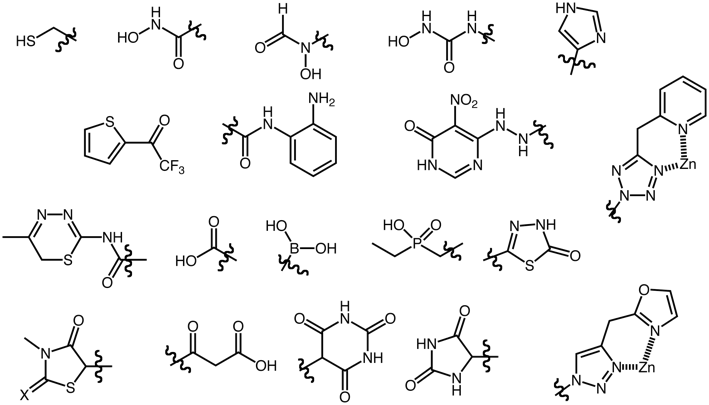
Soft ions like zinc tend to have less rigid (4- or 5-) coordination to sulphur or nitrogen.
Iron can take various oxidation states and thus coordination states.
And may change oxidation state during reaction.
Worth reading:- Structural and Functional Aspects of Metal Sites in Biology R Holm, P Kennepohl, and E I Solomon Chem. Rev. 1996, 96, 2239-2314
Examples of Zinc ligands
Examples of Magnesium ligands
Many thanks to all those who sent examples
Back to Drug Discovery Resources
