Privileged Structures
The term "privileged structures" was first coined by Ben Evans DOI: 10.1021/jm00120a002 who recognised the potential of certain regularly occurring structural motifs as templates for derivatization to discovery novel ligands for binding to proteins. In this seminal paper they identified a benzodiazepine and substituted indole as key structures in their work to yield CCK antagonists.
Two very popular privileged structures are N-benzyl piperidine and N-benzyl piperazine. They offer a variety of different interactions (pi-stacking, hydrophobic, electrostatic) with a relatively well defined 3D structure.
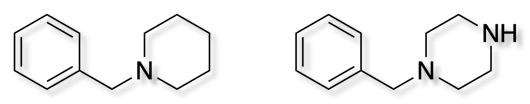
A recent publication gives a very nice summary of their use in drug discovery DOI.
Abstract The N-benzyl piperidine (N-BP) structural motif is commonly employed in drug discovery due to its structural flexibility and three-dimensional nature. Medicinal chemists frequently utilize the N-BP motif as a versatile tool to fine-tune both efficacy and physicochemical properties in drug development. It provides crucial cation-π interactions with the target protein and also serves as a platform for optimizing stereochemical aspects of potency and toxicity. This motif is found in numerous approved drugs and clinical/preclinical candidates. This review focuses on the applications of the N-BP motif in drug discovery campaigns, emphasizing its role in imparting medicinally relevant properties. We provide an overview of approved drugs, the clinical and preclinical pipeline, and discuss its utility for specific therapeutic targets and indications, along with potential challenges.